Oxidizing agent
The international pictogram for oxidizing chemicals.

Dangerous goods label for oxidizing agents
In chemistry, an oxidizing agent (oxidant, oxidizer) is a substance that has the ability to oxidize other substances — in other words to cause them to lose electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.
In one sense, an oxidizing agent is a chemical species that undergoes a chemical reaction that removes one or more electrons from another atom. In that sense, it is one component in an oxidation–reduction (redox) reaction. In the second sense, an oxidizing agent is a chemical species that transfers electronegative atoms, usually oxygen, to a substrate. Combustion, many explosives, and organic redox reactions involve atom-transfer reactions.
Contents
1 Electron acceptors
2 Atom-transfer reagents
2.1 Common oxidizing agents
3 Dangerous materials definition
4 Common oxidizing agents and their products
5 See also
6 References
7 External links
Electron acceptors
Electron acceptors participate in electron-transfer reactions. In this context, the oxidizing agent is called an electron acceptor and the reducing agent is called an electron donor. A classic oxidizing agent is the ferrocenium ion Fe(C
5H
5)+
2, which accepts an electron to form Fe(C5H5)2. One of the strongest acceptors commercially available is "Magic blue", the radical cation derived from N(C6H4-4-Br)3.[1]
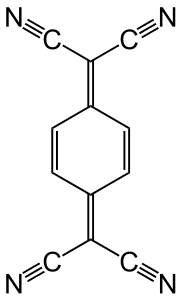
Tetracyanoquinodimethane is an organic electron-acceptor.
Extensive tabulations of ranking the electron accepting properties of various reagents (redox potentials) are available, see Standard electrode potential (data page).
Atom-transfer reagents
In more common usage, an oxidising agent transfers oxygen atoms to a substrate. In this context, the oxidising agent can be called an oxygenation reagent or oxygen-atom transfer (OAT) agent.[2] Examples include MnO−
4 (permanganate), CrO2−
4 (chromate), OsO4 (osmium tetroxide), and especially ClO−
4 (perchlorate). Notice that these species are all oxides.
In some cases, these oxides can also serve as electron acceptors, as illustrated by the conversion of MnO−
4 to MnO2−
4, manganate.
Common oxidizing agents
Oxygen (O2)
Ozone (O3)
Hydrogen peroxide (H2O2) and other inorganic peroxides, Fenton's reagent
Fluorine (F2), chlorine (Cl2), and other halogens
Nitric acid (HNO3) and nitrate compounds
Sulfuric acid (H2SO4)
Peroxydisulfuric acid (H2S2O8)
Peroxymonosulfuric acid (H2SO5)
Chlorite, chlorate, perchlorate, and other analogous halogen compounds
Hypochlorite and other hypohalite compounds, including household bleach (NaClO)- Hexavalent chromium compounds such as chromic and dichromic acids and chromium trioxide, pyridinium chlorochromate (PCC), and chromate/dichromate compounds
Permanganate compounds such as potassium permanganate- Sodium perborate
Nitrous oxide (N2O), Nitrogen dioxide/Dinitrogen tetroxide (NO2 / N2O4)
Potassium nitrate (KNO3), the oxidizer in black powder- Sodium bismuthate
Dangerous materials definition
The dangerous materials definition of an oxidizing agent is a substance that can cause or contribute to the combustion of other material.[3] By this definition some materials that are classified as oxidising agents by analytical chemists are not classified as oxidising agents in a dangerous materials sense. An example is potassium dichromate, which does not pass the dangerous goods test of an oxidising agent.
The U.S. Department of Transportation defines oxidizing agents specifically. There are two definitions for oxidizing agents governed under DOT regulations. These two are Class 5; Division 5.1(a)1 and Class 5; Division 5.1(a)2. Division 5.1 "means a material that may, generally by yielding oxygen, cause or enhance the combustion of other materials." Division 5.(a)1 of the DOT code applies to solid oxidizers "if, when tested in accordance with the UN Manual of Tests and Criteria (IBR, see § 171.7 of this subchapter), its mean burning time is less than or equal to the burning time of a 3:7 potassium bromate/cellulose mixture." 5.1(a)2 of the DOT code applies to liquid oxidizers "if, when tested in accordance with the UN Manual of Tests and Criteria, it spontaneously ignites or its mean time for a pressure rise from 690 kPa to 2070 kPa gauge is less than the time of a 1:1 nitric acid (65 percent)/cellulose mixture."[4]
Common oxidizing agents and their products
| Agent | Product(s) |
|---|---|
| O2oxygen | Various, including the oxides H2O and CO2 |
| O3ozone | Various, including ketones, aldehydes, and H2O; see ozonolysis |
| F2fluorine | F− |
| Cl2chlorine | Cl− |
| Br2bromine | Br− |
| I2iodine | I−, I− 3 |
| ClO−hypochlorite | Cl−, H2O |
ClO− 3 chlorate | Cl−, H2O |
| HNO3nitric acid | NO nitric oxide NO2nitrogen dioxide |
| SO2sulphur dioxide | S sulphur (Claus process, ultramarine production, more commonly reducing agent) |
| Hexavalent chromium CrO3chromium trioxide CrO2− 4 chromate Cr 2O2− 7 dichromate | Cr3+, H2O |
MnO− 4 permanganate MnO2− 4 manganate | Mn2+ (acidic) or MnO2 (basic) |
RuO4displaystyle ce RuO4  ruthenium tetroxide ruthenium tetroxideOsO4displaystyle ce OsO4  osmium tetroxide osmium tetroxide | in organic lab scale synthesis |
| H2O2, other peroxides | Various, including oxides and H2O |
Tl(III) thallic compounds thallium | Tl(I) thallous compounds, in organic lab scale synthesis |
See also
- Combustion
- Dye
- Electrosynthesis
- Organic oxidation
- Organic redox reaction
- Reducing agent
References
^ N. G. Connelly, W. E. Geiger (1996). "Chemical Redox Agents for Organometallic Chemistry". Chemical Reviews. 96 (2): 877–910. doi:10.1021/cr940053x. PMID 11848774..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 0-471-72091-7
^ Australian Dangerous Goods Code, 6th Edition
^ 49 CFR 172.127 General Requirements for Shipments and Packagings; Subpart D
External links
 Media related to Oxidizing agents at Wikimedia Commons
Media related to Oxidizing agents at Wikimedia Commons

