Opioid
| Opioid | |
|---|---|
| Drug class | |
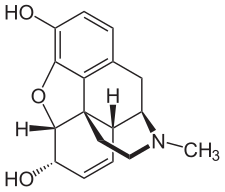 Chemical structure of morphine, the prototypical opioid.[1] | |
| Class identifiers | |
| Use | Pain relief |
| ATC code | N02A |
| Mode of action | Opioid receptor |
| External links | |
| MeSH | D000701 |
| In Wikidata | |
Opioids are narcotics that act on opioid receptors to produce morphine-like effects.[2] Medically they are primarily used for pain relief, including anesthesia.[3] Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, suppressing cough, suppressing opioid induced constipation,[3] as well as for executions in the United States. Extremely potent opioids such as carfentanil are only approved for veterinary use.[4] Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal.[5]
Side effects of opioids may include itchiness, sedation, nausea, respiratory depression, constipation, and euphoria. Tolerance and dependence will develop with continuous use, requiring increasing doses and leading to a withdrawal syndrome upon abrupt discontinuation. The euphoria attracts recreational use and frequent, escalating recreational use of opioids typically results in addiction. An overdose or concurrent use with other depressant drugs commonly results in death from respiratory depression.[6]
Opioids act by binding to opioid receptors, which are found principally in the central and peripheral nervous system and the gastrointestinal tract. These receptors mediate both the psychoactive and the somatic effects of opioids. Opioid drugs include partial agonists, like the anti-diarrhea drug loperamide and antagonists like naloxegol for opioid-induced constipation, which do not cross the blood-brain barrier, but can displace other opioids from binding to those receptors.
Because opioids are addictive and may result in fatal overdose, most are controlled substances. In 2013, between 28 and 38 million people used opioids illicitly (0.6% to 0.8% of the global population between the ages of 15 and 65).[7] In 2011, an estimated 4 million people in the United States used opioids recreationally or were dependent on them.[8] As of 2015, increased rates of recreational use and addiction are attributed to over-prescription of opioid medications and inexpensive illicit heroin.[9][10][11] Conversely, fears about over-prescribing, exaggerated side effects and addiction from opioids are similarly blamed for under-treatment of pain.[12][13]
.mw-parser-output .toclimit-2 .toclevel-1 ul,.mw-parser-output .toclimit-3 .toclevel-2 ul,.mw-parser-output .toclimit-4 .toclevel-3 ul,.mw-parser-output .toclimit-5 .toclevel-4 ul,.mw-parser-output .toclimit-6 .toclevel-5 ul,.mw-parser-output .toclimit-7 .toclevel-6 uldisplay:none
Contents
1 Terminology
2 Medical uses
2.1 Pain
2.1.1 Acute pain
2.1.2 Chronic non-cancer pain
2.2 Other
2.2.1 Cough
2.2.2 Diarrhea and constipation
2.2.3 Shortness of breath
3 Adverse effects
3.1 Reinforcement disorders
3.1.1 Tolerance
3.1.2 Physical dependence
3.1.3 Addiction
3.2 Nausea and vomiting
3.3 Drowsiness
3.4 Itching
3.5 Constipation
3.5.1 Treatment
3.6 Respiratory depression
3.7 Opioid-induced hyperalgesia
3.8 Other adverse effects
3.8.1 Low sex hormone levels
3.8.2 Disruption of work
3.8.3 Increased accident-proneness
3.8.4 Rare side effects
4 Interactions
4.1 With other depressant drugs
4.2 Opioid antagonist
5 Pharmacology
5.1 Functional selectivity
5.2 Opioid comparison
5.3 Binding profiles
6 Usage
7 History
7.1 Naturally occurring opioids
7.2 Laudanum
7.3 The opium trade
7.4 Morphine
7.5 Codeine
7.6 Semisynthetic and synthetic opioids
7.7 Criminalization and medical use
8 Society and culture
8.1 Definition
8.2 Efforts to reduce abuse in the US
8.3 Global shortages
8.4 Recreational use
9 Classification
9.1 Endogenous opioids
9.2 Opium alkaloids and derivatives
9.2.1 Opium alkaloids
9.2.2 Esters of morphine
9.2.3 Ethers of morphine
9.2.4 Semi-synthetic alkaloid derivatives
9.3 Synthetic opioids
9.3.1 Anilidopiperidines
9.3.2 Phenylpiperidines
9.3.3 Diphenylpropylamine derivatives
9.3.4 Benzomorphan derivatives
9.3.5 Oripavine derivatives
9.3.6 Morphinan derivatives
9.3.7 Others
9.4 Allosteric modulators
9.5 Opioid antagonists
9.6 Tables of opioids
9.6.1 Table of morphinan opioids
9.6.2 Table of non-morphinan opioids
10 See also
11 References
12 External links
Terminology
Opioids include opiates, an older term that refers to such drugs derived from opium, including morphine itself.[14] Other opioids are semi-synthetic and synthetic drugs such as hydrocodone, oxycodone and fentanyl; antagonist drugs such as naloxone; and endogenous peptides such as the endorphins.[15] The terms opiate and narcotic are sometimes encountered as synonyms for opioid. Opiate is properly limited to the natural alkaloids found in the resin of the opium poppy although some include semi-synthetic derivatives.[14][16]Narcotic, derived from words meaning 'numbness' or 'sleep', as an American legal term, refers to cocaine and opioids, and their source materials; it is also loosely applied to any illegal or controlled psychoactive drug.[17][18] In some jurisdictions all controlled drugs are legally classified as narcotics. The term can have pejorative connotations and its use is generally discouraged where that is the case.[19][20]
Medical uses
Pain
The weak opioid codeine, in low doses and combined with one or more other drugs, is commonly available without a prescription[21] and can be used to treat mild pain.[22] Other opioids are usually reserved for the relief of moderate to severe pain.[22]
Acute pain
Opioids are effective for the treatment of acute pain (such as pain following surgery).[23] For immediate relief of moderate to severe acute pain opioids are frequently the treatment of choice due to their rapid onset, efficacy and reduced risk of dependence. However a new report showed a clear risk of prolonged opioid use when opioid analgesics are initiated for an acute pain management following surgery or trauma.[24] They have also been found to be important in palliative care to help with the severe, chronic, disabling pain that may occur in some terminal conditions such as cancer, and degenerative conditions such as rheumatoid arthritis. In many cases opioids are a successful long-term care strategy for those with chronic cancer pain.
Chronic non-cancer pain
Guidelines have suggested that the risk of opioids is likely greater than their benefits when used for most non-cancer chronic conditions including headaches, back pain, and fibromyalgia.[25] Thus they should be used cautiously in chronic non-cancer pain.[26] If used the benefits and harms should be reassessed at least every three months.[27]
In treating chronic pain, opioids are an option to be tried after other less risky pain relievers have been considered, including paracetamol/acetaminophen or NSAIDs like ibuprofen or naproxen.[28] Some types of chronic pain, including the pain caused by fibromyalgia or migraine, are preferentially treated with drugs other than opioids.[29][30] The efficacy of using opioids to lessen chronic neuropathic pain is uncertain.[31]
Opioids are contraindicated as a first-line treatment for headache because they impair alertness, bring risk of dependence, and increase the risk that episodic headaches will become chronic.[32] Opioids can also cause heightened sensitivity to headache pain.[32] When other treatments fail or are unavailable, opioids may be appropriate for treating headache if the patient can be monitored to prevent the development of chronic headache.[32]
Opioids are being used more frequently in the management of non-malignant chronic pain.[33][34][35] This practice has now led to a new and growing problem with addiction and misuse of opioids.[26][36] Because of various negative effects the use of opioids for long term management of chronic pain is not indicated unless other less risky pain relievers have been found ineffective. Chronic pain which occurs only periodically, such as that from nerve pain, migraines, and fibromyalgia, frequently is better treated with medications other than opioids.[29]Paracetamol and nonsteroidal anti-inflammatory drugs including ibuprofen and naproxen are considered safer alternatives.[37] They are frequently used combined with opioids, such as paracetamol combined with oxycodone (Percocet) and ibuprofen combined with hydrocodone (Vicoprofen), which boosts the pain relief but is also intended to deter recreational use.[38][39]
Other
Cough
Codeine was once viewed as the "gold standard" in cough suppressants, but this position is now questioned.[40] Some recent placebo-controlled trials have found that it may be no better than a placebo for some causes including acute cough in children.[41][42] Thus, it is not recommended for children.[42] Additionally, there is no evidence that hydrocodone is useful in children.[43] Similarly, a 2012 Dutch guideline regarding the treatment of acute cough does not recommend its use.[44] (The opioid analogue dextromethorphan, long claimed to be as effective a cough suppressant as codeine,[45] has similarly demonstrated little benefit in several recent studies.[46])
Low dose morphine may help chronic cough but its use is limited by side effects.[47]
Diarrhea and constipation
In cases of diarrhea-predominate irritable bowel syndrome, opioids may be used to suppress diarrhea. Loperamide is a peripherally selective opioid available without a prescription used to suppress diarrhea.
The ability to suppress diarrhea also produces constipation when opioids are used beyond several weeks.[48]Naloxegol, a peripherally-selective opioid antagonist is now available to treat opioid induced constipation.[49]
Shortness of breath
Opioids may help with shortness of breath particularly in advanced diseases such as cancer and COPD among others.[50][51]
Adverse effects
.mw-parser-output .quoteboxbackground-color:#F9F9F9;border:1px solid #aaa;box-sizing:border-box;padding:10px;font-size:88%.mw-parser-output .quotebox.floatleftmargin:0.5em 1.4em 0.8em 0.mw-parser-output .quotebox.floatrightmargin:0.5em 0 0.8em 1.4em.mw-parser-output .quotebox.centeredmargin:0.5em auto 0.8em auto.mw-parser-output .quotebox.floatleft p,.mw-parser-output .quotebox.floatright pfont-style:inherit.mw-parser-output .quotebox-titlebackground-color:#F9F9F9;text-align:center;font-size:larger;font-weight:bold.mw-parser-output .quotebox-quote.quoted:beforefont-family:"Times New Roman",serif;font-weight:bold;font-size:large;color:gray;content:" “ ";vertical-align:-45%;line-height:0.mw-parser-output .quotebox-quote.quoted:afterfont-family:"Times New Roman",serif;font-weight:bold;font-size:large;color:gray;content:" ” ";line-height:0.mw-parser-output .quotebox .left-alignedtext-align:left.mw-parser-output .quotebox .right-alignedtext-align:right.mw-parser-output .quotebox .center-alignedtext-align:center.mw-parser-output .quotebox citedisplay:block;font-style:normal@media screen and (max-width:360px).mw-parser-output .quoteboxmin-width:100%;margin:0 0 0.8em!important;float:none!important
Common and short term
Itch[52]
Nausea[52]- Vomiting[52]
Constipation[52]
Drowsiness[52]
Dry mouth[52]
Other
- Cognitive effects
- Opioid dependence
- Dizziness
- Decreased sex drive
- Impaired sexual function
- Decreased testosterone levels
- Depression
- Immunodeficiency
- Increased pain sensitivity
- Irregular menstruation
- Increased risk of falls
- Slowed breathing
- Coma
In older adults, opioid use is associated with increased adverse effects such as "sedation, nausea, vomiting, constipation, urinary retention, and falls".[53] As a result, older adults taking opioids are at greater risk for injury.[54] Opioids do not cause any specific organ toxicity, unlike many other drugs, such as aspirin and paracetamol. They are not associated with upper gastrointestinal bleeding and kidney toxicity.[55]
Research suggests that when methadone is used long-term it can build up unpredictably in the body and lead to potentially deadly slowed breathing.[56][57] Used medically, approaching toxicity goes unrecognized because the pain medication effect ends long before the drug's elimination half-life.[58] According to the USCDC, methadone was involved in 31% of opioid related deaths in the US between 1999-2010 and 40% as the sole drug involved, far higher than other opioids.[59] Studies of long term opioids have found that may stop them and minor side effects were common.[60] Addiction occurred in about 0.3%.[60] In the United States in 2016 opioid overdose resulted in the death of 1.7 in 10,000 people.[61]
In the US charts below many deaths involve multiple opioids:
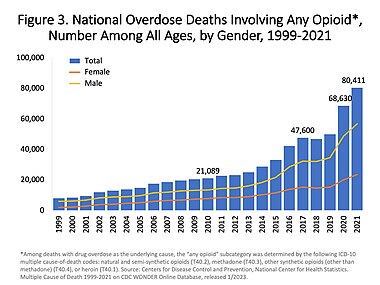
US yearly deaths from all opioid drugs. Included in this number are opioid analgesics, along with heroin and illicit synthetic opioids.[62]
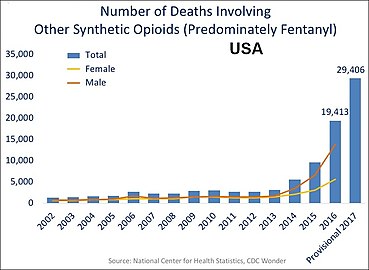
US yearly deaths involving other synthetic opioids, predominately Fentanyl.[62]
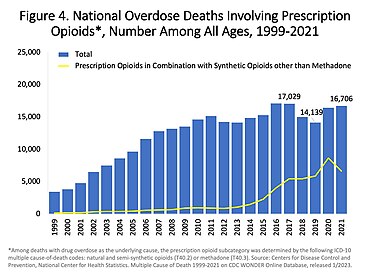
US yearly deaths involving prescription opioids. Non-methadone synthetics is a category dominated by illegally acquired fentanyl, and has been excluded.[62]

US yearly overdose deaths involving heroin.[62]

Fentanyl. 2 mg. A lethal dose in most people.[63]
Reinforcement disorders
Tolerance
Tolerance is a process characterized by neuroadaptations that result in reduced drug effects. While receptor upregulation may often play an important role other mechanisms are also known.[64] Tolerance is more pronounced for some effects than for others; tolerance occurs slowly to the effects on mood, itching, urinary retention, and respiratory depression, but occurs more quickly to the analgesia and other physical side effects. However, tolerance does not develop to constipation or miosis (the constriction of the pupil of the eye to less than or equal to two millimeters). This idea has been challenged, however, with some authors arguing that tolerance does develop to miosis.[65]
Tolerance to opioids is attenuated by a number of substances, including:
calcium channel blockers[66][67][68]
intrathecal magnesium[69][70] and zinc[71]
NMDA antagonists, such as dextromethorphan, ketamine,[72] and memantine.[73]
cholecystokinin antagonists, such as proglumide[74][75][76]- Newer agents such as the phosphodiesterase inhibitor ibudilast have also been researched for this application.[77]
Tolerance is a physiologic process where the body adjusts to a medication that is frequently present, usually requiring higher doses of the same medication over time to achieve the same effect. It is a common occurrence in individuals taking high doses of opioids for extended periods, but does not predict any relationship to misuse or addiction.
Physical dependence
Physical dependence is the physiological adaptation of the body to the presence of a substance, in this case opioid medication. It is defined by the development of withdrawal symptoms when the substance is discontinued, when the dose is reduced abruptly or, specifically in the case of opioids, when an antagonist (e.g., naloxone) or an agonist-antagonist (e.g., pentazocine) is administered. Physical dependence is a normal and expected aspect of certain medications and does not necessarily imply that the patient is addicted.
The withdrawal symptoms for opiates may include severe dysphoria, craving for another opiate dose, irritability, sweating, nausea, rhinorrea, tremor, vomiting and myalgia. Slowly reducing the intake of opioids over days and weeks can reduce or eliminate the withdrawal symptoms.[78] The speed and severity of withdrawal depends on the half-life of the opioid; heroin and morphine withdrawal occur more quickly than methadone withdrawal. The acute withdrawal phase is often followed by a protracted phase of depression and insomnia that can last for months. The symptoms of opioid withdrawal can be treated with other medications, such as clonidine.[79] Physical dependence does not predict drug misuse or true addiction, and is closely related to the same mechanism as tolerance. While there is anecdotal claims of benefit with ibogaine, data to support its use in substance dependence is poor.[80]
Addiction
Drug addiction is a complex set of behaviors typically associated with misuse of certain drugs, developing over time and with higher drug dosages. Addiction includes psychological compulsion, to the extent that the sufferer persists in actions leading to dangerous or unhealthy outcomes. Opioid addiction includes insufflation or injection, rather than taking opioids orally as prescribed for medical reasons.[78]
In European nations such as Austria, Bulgaria, and Slovakia, slow release oral morphine formulations are used in opiate substitution therapy (OST) for patients who do not well tolerate the side effects of buprenorphine or methadone. In other European countries including the UK, this is also legally used for OST although on a varying scale of acceptance.
Tamper-release formulations of time-controlled preparations of medications are intended to curb abuse and addiction rates while trying to still provide legitimate pain relief and ease of use to pain patients. Questions remain, however, about the efficacy and safety of these types of preparations. Further tamper resistant medications are currently under consideration with trials for market approval by the FDA.[81][82]
The amount of evidence available only permits making a weak conclusion, but it suggests that a physician properly managing opioid use in patients with no history of substance dependence or substance abuse can give long-term pain relief with little risk of developing addiction, abuse, or other serious side effects.[60]
Problems with opioids include the following:
- Some people find that opioids do not relieve all of their pain.[83]
- Some people find that opioids side effects cause problems which outweigh the therapy's benefit[60]
- Some people build tolerance to opioids over time. This requires them to increase their drug dosage to maintain the benefit, and that in turn also increases the unwanted side effects.[60]
- Long-term opioid use can cause opioid-induced hyperalgesia, which is a condition in which the patient has increased sensitivity to pain.[84]
All of the opioids can cause side effects.[52] Common adverse reactions in patients taking opioids for pain relief include nausea and vomiting, drowsiness, itching, dry mouth, dizziness, and constipation.[52][78]
Nausea and vomiting
Tolerance to nausea occurs within 7–10 days, during which antiemetics (e.g. low dose haloperidol once at night) are very effective.[citation needed] Due to severe side effects such as tardive dyskinesia, haloperidol is now rarely used. A related drug, prochlorperazine is more often used, although it has similar risks. Stronger antiemetics such as ondansetron or tropisetron are sometimes used when nausea is severe or continuous and disturbing, despite their greater cost. A less expensive alternative is dopamine antagonists such as domperidone and metoclopramide. Domperidone does not cross the blood–brain barrier and produce adverse central antidopaminergic effects, but blocks opioid emetic action in the chemoreceptor trigger zone. (The drug is not available in the U.S.) Some antihistamines with anticholinergic properties (e.g. orphenadrine or diphenhydramine) may also be effective. The first-generation antihistamine hydroxyzine is very commonly used, with the added advantages of not causing movement disorders, and also possessing analgesic-sparing properties. Δ9-tetrahydrocannabinol relieves nausea and vomiting;[85][86] it also produces analgesia that may allow lower doses of opioids with reduced nausea and vomiting.[87][88]
- 5-HT3 antagonists (e.g. ondansetron)
- Dopamine antagonists (e.g. domperidone)
- Anti-cholinergic antihistamines (e.g. diphenhydramine)
- Δ9-tetrahydrocannabinol (e.g. dronabinol)
Vomiting is due to gastric stasis (large volume vomiting, brief nausea relieved by vomiting, oesophageal reflux, epigastric fullness, early satiation), besides direct action on the chemoreceptor trigger zone of the area postrema, the vomiting centre of the brain. Vomiting can thus be prevented by prokinetic agents (e.g. domperidone or metoclopramide). If vomiting has already started, these drugs need to be administered by a non-oral route (e.g. subcutaneous for metoclopramide, rectally for domperidone).
- Prokinetic agents (e.g. domperidone)
- Anti-cholinergic agents (e.g. orphenadrine)
Drowsiness
Tolerance to drowsiness usually develops over 5–7 days, but if troublesome, switching to an alternative opioid often helps. Certain opioids such as fentanyl, morphine and diamorphine (heroin) tend to be particularly sedating, while others such as oxycodone, tilidine and meperidine (pethidine) tend to produce comparatively less sedation, but individual patients responses can vary markedly and some degree of trial and error may be needed to find the most suitable drug for a particular patient. Otherwise, treatment with CNS stimulants is generally effective.[89][90]
- Stimulants (e.g. caffeine, modafinil, amphetamine, methylphenidate)
Itching
Itching tends not to be a severe problem when opioids are used for pain relief, but antihistamines are useful for counteracting itching when it occurs. Non-sedating antihistamines such as fexofenadine are often preferred as they avoid increasing opioid induced drowsiness. However, some sedating antihistamines such as orphenadrine can produce a synergistic pain relieving effect permitting smaller doses of opioids be used. Consequently, several opioid/antihistamine combination products have been marketed, such as Meprozine (meperidine/promethazine) and Diconal (dipipanone/cyclizine), and these may also reduce opioid induced nausea.
- Antihistamines (e.g. fexofenadine)
Constipation
Opioid-induced constipation (OIC) develops in 90 to 95% of people taking opioids long-term.[91] Since tolerance to this problem does not develop readily, most people on long-term opioids need to take a laxative or enemas.[92] While all opioids cause constipation, there are some differences between drugs, with studies suggesting tramadol, tapentadol, methadone and fentanyl may cause relatively less constipation, while with codeine, morphine, oxycodone or hydromorphone constipation may be comparatively more severe. Opioid rotation is commonly used to try and minimise the impact of constipation in long-term users.[93][94]
Treatment
Treatment of OIC is successional and dependent on severity.[95] The first mode of treatment is non-pharmacological, and includes lifestyle modifications like increasing dietary fiber, fluid intake (around 1.5 L (51 US fl oz) per day), and physical activity.[95] If non-pharmacological measures are ineffective, laxatives, including stool softeners (e.g., docusate), bulk-forming laxatives (e.g., fiber supplements), stimulant laxatives (e.g., bisacodyl, senna), and/or enemas, may be used.[95] A common laxative regimen for OIC is the combination of docusate and bisacodyl.[95][96][97]Osmotic laxatives, including lactulose, polyethylene glycol, and milk of magnesia (magnesium hydroxide), as well as mineral oil (a lubricant laxative), are also commonly used for OIC.[96][97]
If laxatives are insufficiently effective (which is often the case),[98] opioid formulations or regimens that include a peripherally-selective opioid antagonist, such as methylnaltrexone bromide, naloxegol, alvimopan, or naloxone (as in oxycodone/naloxone), may be tried.[95][97][99] A 2008 Cochrane review found that the evidence was tentative for alvimopan, naloxone, or methylnaltrexone bromide.[100][needs update]
Respiratory depression
Respiratory depression is the most serious adverse reaction associated with opioid use, but it usually is seen with the use of a single, intravenous dose in an opioid-naïve patient. In patients taking opioids regularly for pain relief, tolerance to respiratory depression occurs rapidly, so that it is not a clinical problem. Several drugs have been developed which can partially block respiratory depression, although the only respiratory stimulant currently approved for this purpose is doxapram, which has only limited efficacy in this application.[101][102] Newer drugs such as BIMU-8 and CX-546 may be much more effective.[103][104][105][non-primary source needed]
- Respiratory stimulants: carotid chemoreceptor agonists (e.g. doxapram), 5-HT4 agonists (e.g. BIMU8), δ-opioid agonists (e.g. BW373U86) and AMPAkines (e.g. CX717) can all reduce respiratory depression caused by opioids without affecting analgesia, but most of these drugs are only moderately effective or have side effects which preclude use in humans. 5-HT1A agonists such as 8-OH-DPAT and repinotan also counteract opioid-induced respiratory depression, but at the same time reduce analgesia, which limits their usefulness for this application.
- Opioid antagonists (e.g. naloxone, nalmefene, diprenorphine)
Opioid-induced hyperalgesia
Opioid-induced hyperalgesia – where individuals using opioids to relieve pain paradoxically experience more pain as a result of that medication – has been observed in some people. This phenomenon, although uncommon, is seen in some people receiving palliative care, most often when dose is increased rapidly.[106][107] If encountered, rotation between several different opioid pain medications may decrease the development of increased pain.[108][109] Opioid induced hyperalgesia more commonly occurs with chronic use or brief high doses but some research suggests that it may also occur with very low doses.[110][111]
Side effects such as hyperalgesia and allodynia, sometimes accompanied by a worsening of neuropathic pain, may be consequences of long-term treatment with opioid analgesics, especially when increasing tolerance has resulted in loss of efficacy and consequent progressive dose escalation over time. This appears to largely be a result of actions of opioid drugs at targets other than the three classic opioid receptors, including the nociceptin receptor, sigma receptor and Toll-like receptor 4, and can be counteracted in animal models by antagonists at these targets such as J-113,397, BD-1047 or (+)-naloxone respectively.[112] No drugs are currently approved specifically for counteracting opioid-induced hyperalgesia in humans and in severe cases the only solution may be to discontinue use of opioid analgesics and replace them with non-opioid analgesic drugs. However, since individual sensitivity to the development of this side effect is highly dose dependent and may vary depending which opioid analgesic is used, many patients can avoid this side effect simply through dose reduction of the opioid drug (usually accompanied by the addition of a supplemental non-opioid analgesic), rotating between different opioid drugs, or by switching to a milder opioid with a mixed mode of action that also counteracts neuropathic pain, particularly tramadol or tapentadol.[113][114][115]
NMDA receptor antagonists such as ketamine
SNRIs such as milnacipran
Anticonvulsants such as gabapentin or pregabalin
Other adverse effects
Low sex hormone levels
Clinical studies have consistently associated medical and recreational opioid use with hypogonadism (low sex hormone levels) in different sexes. The effect is dose-dependent. Most studies suggest that the majority (perhaps as much as 90%) of chronic opioid users suffer from hypogonadism. Opioids can also interfere with menstruation in women by limiting the production of luteinizing hormone (LH). Opioid-induced hypogonadism likely causes the strong association of opioid use with osteoporosis and bone fracture, due to deficiency in estradiol. It also may increase pain and thereby interfere with the intended clinical effect of opioid treatment. Opioid-induced hypogonadism is likely caused by their agonism of opioid receptors in the hypothalamus and the pituitary gland.[citation needed] One study found that the depressed testosterone levels of heroin addicts returned to normal within one month of abstinence, suggesting that the effect is readily reversible and is not permanent.[citation needed] As of 2013[update], the effect of low-dose or acute opioid use on the endocrine system is unclear.[116][117][118]
Disruption of work
Use of opioids may be a risk factor for failing to return to work.[119][120]
Persons performing any safety-sensitive task should not use opioids.[121] Health care providers should not recommend that workers who drive or use heavy equipment including cranes or forklifts treat chronic or acute pain with opioids.[121] Workplaces which manage workers who perform safety-sensitive operations should assign workers to less sensitive duties for so long as those workers are treated by their physician with opioids.[121]
People who take opioids long term have increased likelihood of being unemployed.[122] Taking opioids may further disrupt the patient's life and the adverse effects of opioids themselves can become a significant barrier to patients having an active life, gaining employment, and sustaining a career.
In addition, lack of employment may be a predictor of aberrant use of prescription opioids.[123]
Increased accident-proneness
Opioid use may increase accident-proneness. Opioids may increase risk of traffic accidents[124][125] and accidental falls.[126]
Rare side effects
Infrequent adverse reactions in patients taking opioids for pain relief include: dose-related respiratory depression (especially with more potent opioids), confusion, hallucinations, delirium, urticaria, hypothermia, bradycardia/tachycardia, orthostatic hypotension, dizziness, headache, urinary retention, ureteric or biliary spasm, muscle rigidity, myoclonus (with high doses), and flushing (due to histamine release, except fentanyl and remifentanil).[78]
Both therapeutic and chronic use of opioids can compromise the function of the immune system. Opioids decrease the proliferation of macrophage progenitor cells and lymphocytes, and affect cell differentiation (Roy & Loh, 1996). Opioids may also inhibit leukocyte migration. However the relevance of this in the context of pain relief is not known.
Interactions
Physicians treating patients using opioids in combination with other drugs keep continual documentation that further treatment is indicated and remain aware of opportunities to adjust treatment if the patient's condition changes to merit less risky therapy.[127]
With other depressant drugs

US. Top line represents the number of benzodiazepine deaths that also involved opioids. Bottom line represents benzodiazepine deaths that did not involve opioids.[62]
The concurrent use of opioids with other depressant drugs such as benzodiazepines or ethanol increases the rates of adverse events and overdose.[127] As with an overdose of opioid alone, the combination of an opioid and another depressant may precipitate respiratory depression often leading to death.[128] These risks are lessened with close monitoring by a physician, who may conduct ongoing screening for changes in patient behavior and treatment compliance.[127]
Opioid antagonist
Opioid effects (adverse or otherwise) can be reversed with an opioid antagonist such as naloxone or naltrexone.[129] These competitive antagonists bind to the opioid receptors with higher affinity than agonists but do not activate the receptors. This displaces the agonist, attenuating or reversing the agonist effects. However, the elimination half-life of naloxone can be shorter than that of the opioid itself, so repeat dosing or continuous infusion may be required, or a longer acting antagonist such as nalmefene may be used. In patients taking opioids regularly it is essential that the opioid is only partially reversed to avoid a severe and distressing reaction of waking in excruciating pain. This is achieved by not giving a full dose but giving this in small doses until the respiratory rate has improved. An infusion is then started to keep the reversal at that level, while maintaining pain relief. Opioid antagonists remain the standard treatment for respiratory depression following opioid overdose, with naloxone being by far the most commonly used, although the longer acting antagonist nalmefene may be used for treating overdoses of long-acting opioids such as methadone, and diprenorphine is used for reversing the effects of extremely potent opioids used in veterinary medicine such as etorphine and carfentanil. However, since opioid antagonists also block the beneficial effects of opioid analgesics, they are generally useful only for treating overdose, with use of opioid antagonists alongside opioid analgesics to reduce side effects, requiring careful dose titration and often being poorly effective at doses low enough to allow analgesia to be maintained.
Pharmacology
| Drug | Relative Potency [130] | Nonionized Fraction | Protein Binding | Lipid Solubility [131][132] |
|---|---|---|---|---|
Morphine | 1 | ++ | ++ | ++ |
Pethidine (meperidine) | 0.1 | + | +++ | +++ |
Hydromorphone | 10 | + | +++ | |
Alfentanil | 10–25 | ++++ | ++++ | +++ |
Fentanyl | 75–125 | + | +++ | ++++ |
Remifentanil | 250 | +++ | +++ | ++ |
Sufentanil | 500–1000 | ++ | ++++ | ++++ |
Etorphine | 1000–3000 | |||
Carfentanil | 10000 |
Opioids bind to specific opioid receptors in the nervous system and other tissues. There are three principal classes of opioid receptors, μ, κ, δ (mu, kappa, and delta), although up to seventeen have been reported, and include the ε, ι, λ, and ζ (Epsilon, Iota, Lambda and Zeta) receptors. Conversely, σ (Sigma) receptors are no longer considered to be opioid receptors because their activation is not reversed by the opioid inverse-agonist naloxone, they do not exhibit high-affinity binding for classical opioids, and they are stereoselective for dextro-rotatory isomers while the other opioid receptors are stereo-selective for levo-rotatory isomers. In addition, there are three subtypes of μ-receptor: μ1 and μ2, and the newly discovered μ3. Another receptor of clinical importance is the opioid-receptor-like receptor 1 (ORL1), which is involved in pain responses as well as having a major role in the development of tolerance to μ-opioid agonists used as analgesics. These are all G-protein coupled receptors acting on GABAergic neurotransmission.

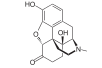
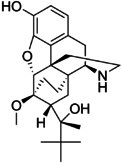
Locants of the morphine molecule
The pharmacodynamic response to an opioid depends upon the receptor to which it binds, its affinity for that receptor, and whether the opioid is an agonist or an antagonist. For example, the supraspinal analgesic properties of the opioid agonist morphine are mediated by activation of the μ1 receptor; respiratory depression and physical dependence by the μ2 receptor; and sedation and spinal analgesia by the κ receptor[citation needed]. Each group of opioid receptors elicits a distinct set of neurological responses, with the receptor subtypes (such as μ1 and μ2 for example) providing even more [measurably] specific responses. Unique to each opioid is its distinct binding affinity to the various classes of opioid receptors (e.g. the μ, κ, and δ opioid receptors are activated at different magnitudes according to the specific receptor binding affinities of the opioid). For example, the opiate alkaloid morphine exhibits high-affinity binding to the μ-opioid receptor, while ketazocine exhibits high affinity to ĸ receptors. It is this combinatorial mechanism that allows for such a wide class of opioids and molecular designs to exist, each with its own unique effect profile. Their individual molecular structure is also responsible for their different duration of action, whereby metabolic breakdown (such as N-dealkylation) is responsible for opioid metabolism.

INTA: selective agonist of KOR-DOR and KOR-MOR heteromers. Does not recruit β-arrestin II. Antinociceptive devoid of aversion, tolerance, and dependence in mice.[133]
Functional selectivity
A new strategy of drug development takes receptor signal transduction into consideration. This strategy strives to increase the activation of desirable signalling pathways while reducing the impact on undesirable pathways. This differential strategy has been given several names, including functional selectivity and biased agonism. The first opioid that was intentionally designed as a biased agonist and placed into clinical evaluation is the drug oliceridine. It displays analgesic activity and reduced adverse effects.[134]
Opioid comparison
Extensive research has been conducted to determine equivalence ratios comparing the relative potency of opioids. Given a dose of an opioid, an equianalgesic table is used to find the equivalent dosage of another. Such tables are used in opioid rotation practices, and to describe an opioid by comparison to morphine, the reference opioid. Equianalgesic tables typically list drug half-lives, and sometimes equianalgesic doses of the same drug by means of administration, such as morphine: oral and intravenous.
Binding profiles
| Compound | MOR | DOR | KOR | Ref | |
|---|---|---|---|---|---|
| 7-Hydroxymitragynine | 13.5 | 155 | 123 | [135] | |
| β-Chlornaltrexamine | 0.90 | 115 | 0.083 | [136] | |
| β-Endorphin | 1.0 | 1.0 | 52 | [136] | |
| β-Funaltrexamine | 0.33 | 48 | 2.8 | [136] | |
| Alazocine | 2.7 | 4.1 | 3.2 | [137] | |
| (−)-Alazocine | 3.0 | 15 | 4.7 | [138] | |
| (+)-Alazocine | 1,900 | 19,000 | 1,600 | [138] | |
| Alfentanil | 39 | 21,200 | ND | [139] | |
| Binaltorphimine | 1.3 | 5.8 | 0.79 | [139] | |
| BNTX | 18 | 0.66 | 55 | [136] | |
| Bremazocine | 0.75 | 2.3 | 0.089 | [136] | |
| (−)-Bremazocine | 0.62 | 0.78 | 0.075 | [139] | |
| Buprenorphine | 4.18 | 25.8 | 12.9 | [140] | |
| Butorphanol | 1.7 | 13 | 7.4 | [138] | |
| BW-3734 | 26 | 0.013 | 17 | [136] | |
| Carfentanil | 0.024 | 3.3 | 43 | [137] | |
| Codeine | 79 | >1,000 | >1,000 | [136] | |
| CTOP | 0.18 | >1,000 | >1,000 | [136] | |
| Cyclazocine | 0.45 | 6.3 | 5.9 | [138] | |
| Cyprodime | 9.4 | 356 | 176 | [139] | |
| DADLE | 16 | 0.74 | >1,000 | [136] | |
| DAMGO | 2.0 | >1,000 | >1,000 | [136] | |
| [D-Ala2]Deltorphin II | >1,000 | 3.3 | >1,000 | [136] | |
| Dermorphin | 0.33 | >1,000 | >1,000 | [136] | |
| (+)-Desmetramadol | 17 | 690 | 1,800 | [141][142] | |
| Dextropropoxyphene | 34.5 | 380 | 1,220 | [140] | |
| Dezocine | 3.6 | 290 | 460 | [137] | |
| Dihydroetorphine | 0.45 | 1.82 | 0.57 | [143] | |
| Diprenorphine | 0.072 | 0.23 | 0.017 | [136] | |
| DPDPE | >1,000 | 14 | >1,000 | [136] | |
| DSLET | 39 | 4.8 | >1,000 | [136] | |
| Dynorphin A | 32 | >1,000 | 0.5 | [136] | |
| Ethylketazocine | 3.1 | 101 | 0.40 | [136] | |
| (−)-Ethylketazocine | 2.3 | 5.2 | 2.2 | [138] | |
| (+)-Ethylketazocine | 2,500 | >10,000 | 1,600 | [138] | |
| Etorphine | 0.23 | 1.4 | 0.13 | [136] | |
| Fentanyl | 0.39 | >1,000 | 255 | [136] | |
| Hydrocodone | 11.1 | 962 | 501 | [140] | |
| Hydromorphone | 0.47 | 18.5 | 24.9 | [137] | |
| ICI-204488 | >1,000 | >1,000 | 0.71 | [136] | |
| Leu-enkephalin | 3.4 | 4.0 | >1,000 | [136] | |
| Levacetylmethadol | 9.86 | 169 | 1,020 | [140] | |
| Lofentanil | 0.68 | 5.5 | 5.9 | [136] | |
| Met-enkephalin | 0.65 | 1.7 | >1,000 | [136] | |
| Metazocine | 3.8 | 44.3 | 13.3 | [137] | |
| Methadone | 1.7 | 435 | 405 | [140] | |
| Dextromethadone | 19.7 | 960 | 1,370 | [140] | |
| Levomethadone | 0.945 | 371 | 1,860 | [140] | |
| Methallorphan | ND | ND | ND | ND | |
| Dextrallorphan | 1,140 | 2,660 | 34.6 | [140] | |
| Levallorphan | 0.213 | 2.18 | 1,100 | [140] | |
| Methorphan | ND | ND | ND | ND | |
| Dextromethorphan | 1,280 | 11,500 | 7,000 | [140] | |
| Levomethorphan | 11.2 | 249 | 225 | [140] | |
| Mitragynine | 7.24 | 60.3 | 1,100 | [135] | |
| Mitragynine pseudoindoxyl | 0.087 | 3.02 | 79.4 | [135] | |
| Morphanol | ND | ND | ND | ND | |
| Dextrorphan | 420 | 7004347000000000000♠34,700 | 7003595000000000000♠5,950 | [140] | |
| Levorphanol | 0.42 | 3.61 | 4.2 | [140] | |
| Morphiceptin | 56 | >1,000 | >1,000 | [136] | |
| Morphine | 1.8 | 90 | 317 | [139] | |
| Morphine, (−)- | 1.24 | 145 | 23.4 | [140] | |
| Morphine, (+)- | >10,000 | >100,000 | >300,000 | [140] | |
| MR-2266 | 1.0 | 3.0 | 0.16 | [137] | |
| Nalbuphine | 11 | >1,000 | 3.9 | [136] | |
| Nalmefene | 0.24 | 16 | 0.083 | [144] | |
| Nalorphine | 0.97 | 148 | 1.1 | [136] | |
| Naloxonazine | 0.054 | 8.6 | 11 | [136] | |
| Naloxone | 1.1 | 16 | 12 | [138] | |
| (−)-Naloxone | 0.93 | 17 | 2.3 | [136] | |
| (+)-Naloxone | >1,000 | >1,000 | >1,000 | [136] | |
| Naltrexone | 1.0 | 149 | 3.9 | [136] | |
| Naltriben | 12 | 0.013 | 13 | [136] | |
| Naltrindole | 64 | 0.02 | 66 | [136] | |
| Norbinaltorphimine | 2.2 | 65 | 0.027 | [136] | |
| Normorphine | 4.0 | 310 | 149 | [139] | |
| Ohmefentanyl | 0.0079 | 10 | 32 | [139] | |
| Oxycodone | 8.69 | 901 | 1,350 | [140] | |
| Oxymorphindole | 111 | 0.7 | 228 | [137] | |
| Oxymorphone | 0.78 | 50 | 137 | [139] | |
| Pentazocine | 5.7 | 31 | 7.2 | [136] | |
| Pethidine (meperidine) | 385 | 7003435000000000000♠4,350 | 5,140 | [139] | |
| Phenazocine | 0.20 | 5.0 | 2.0 | [145] | |
| PLO17 | 30 | >1,000 | >1,000 | [136] | |
| Quadazocine | 0.99 | 2.6 | 0.5 | [146] | |
| Salvinorin A | >10,000 | >10,000 | 16 | [147] | |
| Samidorphan | 0.052 | 2.6 | 0.23 | [148] | |
| SIOM | 33 | 1.7 | >1,000 | [136] | |
| Spiradoline | 21 | >1,000 | 0.036 | [136] | |
| Sufentanil | 0.15 | 50 | 75 | [136] | |
| Tianeptine | 383 | >10,000 | >10,000 | [149] | |
| Tifluadom | 32 | 189 | 2.1 | [138] | |
| Tramadol | 7003212000000000000♠2,120 | 7004577000000000000♠57,700 | 7004427000000000000♠42,700 | [140] | |
| (+)-Tramadol | 7003133000000000000♠1,330 | 7004624000000000000♠62,400 | 7004540000000000000♠54,000 | [140] | |
| (−)-Tramadol | 7004248000000000000♠24,800 | 7005213000000000000♠213,000 | 7004535000000000000♠53,500 | [140] | |
| U-50488 | >1,000 | >1,000 | 0.12 | [136] | |
| U-69593 | >1,000 | >1,000 | 0.59 | [136] | |
| Xorphanol | 0.25 | 1.0 | 0.4 | [146] | |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. Assays were done mostly with cloned or cultured rodent receptors. | |||||
Usage
| Substance | Best estimate | Low estimate | High estimate |
|---|---|---|---|
| Amphetamine- type stimulants | 34.16 | 13.42 | 55.24 |
| Cannabis | 192.15 | 165.76 | 234.06 |
| Cocaine | 18.20 | 13.87 | 22.85 |
| Ecstasy | 20.57 | 8.99 | 32.34 |
| Opiates | 19.38 | 13.80 | 26.15 |
| Opioids | 34.26 | 27.01 | 44.54 |
Opioid prescriptions in the US increased from 76 million in 1991 to 207 million in 2013.[151]
In the 1990s, opioid prescribing increased significantly. Once used almost exclusively for the treatment of acute pain or pain due to cancer, opioids are now prescribed liberally for people experiencing chronic pain. This has been accompanied by rising rates of accidental addiction and accidental overdoses leading to death. According to the International Narcotics Control Board, the United States and Canada lead the per capita consumption of prescription opioids.[152] The number of opioid prescriptions per capita in the United States and Canada is double the consumption in the European Union, Australia, and New Zealand.[153] Certain populations have been affected by the opioid addiction crisis more than others, including First World communities[154] and low-income populations.[155] Public health specialists say that this may result from unavailability or high cost of alternative methods for addressing chronic pain.[156]
History
Naturally occurring opioids

A sample of raw opium
Opioids are among the world's oldest known drugs.[157]
The earliest known evidence of Papaver somniferum in a human archaeological site dates to the Neolithic period around 5,700-5,500 BC. Its seeds have been found at Cueva de los Murciélagos in the Iberian Peninsula and La Marmotta in the Italian Peninsula.[158][159][160]
Use of the opium poppy for medical, recreational, and religious purposes can be traced to the 4th century BC, when ideograms on Sumerians clay tablets mention the use of "Hul Gil", a "plant of joy".[161][162][163]
Opium was known to the Egyptians, and is mentioned in the Ebers Papyrus as an ingredient in a mixture for the soothing of children,[164][163] and for the treatment of breast abscesses.[165]
Opium was also known to the Greeks.[164]
It was valued by Hippocrates (c. 460 – c. 370 BC) and his students for its sleep-inducing properties, and used for the treatment of pain.[166] The Latin saying "Sedare dolorem opus divinum est", trans. "Alleviating pain is the work of the divine", has been variously ascribed to Hippocrates and to Galen of Pergamum.[167] The medical use of opium is later discussed by Pedanius Dioscorides (c. 40 – 90 AD), a Greek physician serving in the Roman army, in his five-volume work, De Materia Medica.[168]
During the Islamic Golden Age, the use of opium was discussed in detail by Avicenna (c. 980 – June 1037 AD) in The Canon of Medicine. The book's five volumes include information on opium's preparation, an array of physical effects, its use to treat a variety of illness, contraindications for its use, its potential danger as a poison and its potential for addiction. Avicenna discouraged opium's use except as a last resort, preferring to address the causes of pain rather than trying to minimize it with analgesics. Many of Avicenna's observations have been supported by modern medical research.[169][164]
Exactly when opium became known in India and China is uncertain, but opium was mentioned in the Chinese medical work K'ai-pao-pen-tsdo (973 AD)[163] By 1590 AD, opium poppies were a staple spring crop in the Subahs of Agra, Oudh and Allahabad in what is now Uttar Pradesh.[170]
The physician Paracelsus (ca.1493–1541) is often credited with reintroducing opium into medical use in Western Europe, during the German Renaissance. He extolled opium's benefits for medical use. He also claimed to have an "arcanum", a pill which he called laudanum, that was superior to all others, particularly when death was to be cheated. ("Ich hab' ein Arcanum — heiss' ich Laudanum, ist über das Alles, wo es zum Tode reichen will.")[171] Later writers have asserted that Paracelsus' recipe for laudanum contained opium, but its composition remains unknown.[171]
Laudanum
The term laudanum was used generically for a useful medicine until the 17th century. After Thomas Sydenham introduced the first liquid tincture of opium, "laudanum" came to mean a mixture of both opium and alcohol.[171]
Sydenham's 1669 recipe for laudanum mixed opium with wine, saffron, clove and cinnamon.[172] Sydenham's laudanum was used widely in both Europe and the Americas until the 20th century.[164][172]
Other popular medicines, based on opium, included Paregoric, a much milder liquid preparation for children; Black-drop, a stronger preparation; and Dover's powder.[172]
The opium trade
Opium became a major colonial commodity, moving legally and illegally through trade networks involving India, the Portuguese, the Dutch, the British and China, among others.[173]
The British East India Company saw the opium trade as an investment opportunity in 1683 AD.[170] In 1773 the Governor of Bengal established a monopoly on the production of Bengal opium, on behalf of the East India Company. The cultivation and manufacture of Indian opium was further centralized and controlled through a series of acts, between 1797 and 1949.[170][174] The British balanced an economic deficit from the importation of Chinese tea by selling Indian opium which was smuggled into China in defiance of Chinese government bans. This led to the First (1839–1842) and Second Opium Wars (1856–1860) between China and Britain.[175][174][173][176]
Morphine
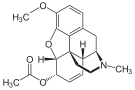
In the 19th century, two major scientific advances were made that had far-reaching effects. Around 1804, German pharmacist Friedrich Sertürner isolated morphine from opium. He described its crystallization, structure, and pharmacological properties in a well-received paper in 1817.[175][177][172][178]
Morphine was the first alkaloid to be isolated from any medicinal plant, the beginning of modern scientific drug discovery.[175][179]
The second advance, nearly fifty years later, was the refinement of the hypodermic needle by Alexander Wood and others. Development of a glass syringe with a subcutaneous needle made it possible to easily administer controlled measurable doses of a primary active compound.[180][172][163][181][182]
Morphine was initially hailed as a wonder drug for its ability to ease pain.[183] It could help people sleep,[175] and had other useful side effects, including control of coughing and diarrhea.[184] It was widely prescribed by doctors, and dispensed without restriction by pharmacists. During the American Civil War, opium and laudanum were used extensively to treat soldiers.[185][183] It was also prescribed frequently for women, for menstrual pain and diseases of a "nervous character".[186]:85
At first it was assumed (wrongly) that this new method of application would not be addictive.[175][186]
Codeine
Codeine was discovered in 1832 by Pierre Jean Robiquet. Robiquet was reviewing a method for morphine extraction, described by Scottish chemist William Gregory (1803-1838). Processing the residue left from Gregory's procedure, Robiquet isolated a crystalline substance from the other active components of opium. He wrote of his discovery: "Here is a new substance found in opium ... We know that morphine, which so far has been thought to be the only active principle of opium, does not account for all the effects and for a long time the physiologists are claiming that there is a gap that has to be filled."[187] His discovery of the alkaloid led to the developmemt of a generation of antitussive and antidiarrheal medicines based on codeine.[188]
Semisynthetic and synthetic opioids
Synthetic opioids were invented, and biological mechanisms for their actions discovered, in the 20th century.[163] Scientists have searched for non-addictive forms of opioids, but have created stronger ones instead. In England Charles Romley Alder Wright developed hundreds of opiate compounds in his search for a nonaddictive opium derivative. In 1874 he became the first person to synthesize diamorphine (heroin), using a process called acetylation which involved boiling morphine with acetic anhydride for several hours.[175]
Heroin received little attention until it was independently synthesized by Felix Hoffmann (1868–1946), working for Heinrich Dreser (1860–1924) at Bayer Laboratories.[189] Dreser brought the new drug to market as an analgesic and a cough treatment for tuberculosis, bronchitis, and asthma in 1898. Bayer ceased production in 1913, after heroin's addictive potential was recognized.[175][190][191]
Several semi-synthetic opioids were developed in Germany in the 1910s. The first, oxymorphone, was synthesized from thebaine, an opioid alkaloid in opium poppies, in 1914.[192]
Next, Martin Freund and Edmund Speyer developed oxycodone, also from thebaine, at the University of Frankfurt in 1916.[193]
In 1920, hydrocodone was prepared by Carl Mannich and Helene Löwenheim, deriving it from codeine. In 1924, hydromorphone was synthesized by adding hydrogen to morphine. Etorphine was synthesized in 1960, from the oripavine in opium poppy straw. Buprenorphine was discovered in 1972.[192]
The first fully synthetic opioid was meperidine (later demerol), found serendipitously by German chemist Otto Eisleb (or Eislib) at IG Farben in 1932.[192] Meperidine was the first opiate to have a structure unrelated to morphine, but with opiate-like properties.[163] Its analgesis effects were discovered by Otto Schaumann in 1939.[192]Gustav Ehrhart and Max Bockmühl, also at IG Farben,
built on the work of Eisleb and Schaumann. They developed "Hoechst 10820" (later methadone) around 1937.[194]
In 1959 the Belgian physician Paul Janssen developed fentanyl, a synthetic drug with 30 to 50 times the potency of heroin.[175][195]
Nearly 150 synthetic opioids are now known.[192]
Criminalization and medical use
Non-clinical use of opium was criminalized in the United States by the Harrison Narcotics Tax Act of 1914, and by many other laws.[196][197] The use of opioids was stigmatizied, and it was seen as a dangerous substance, to be prescribed only as a last resort for dying patients.[175] The Controlled Substances Act of 1970 eventually relaxed the harshness of the Harrison Act.[citation needed]
In the United Kingdom the 1926 report of the Departmental Committee on Morphine and Heroin Addiction under the Chairmanship of the President of the Royal College of Physicians reasserted medical control and established the "British system" of control—which lasted until the 1960s.[198]
In the 1980s the World Health Organization published guidelines for prescribing drugs, including opioids, for different levels of pain. In the U.S., Kathleen Foley and Russell Portenoy became leading advocates for the liberal use of opioids as painkillers for cases of "intractable non-malignant pain".[199][200]
With little or no scientific evidence to support their claims, industry scientists and advocates suggested that chronic pain sufferers would be resistant to addiction.[175][201][199]
The release of OxyContin in 1996 was accompanied by an aggressive marketing campaign promoting the use of opioids for pain relief. Increasing prescription of opioids fueled a growing black market for heroin. Between 2000 and 2014 there was an "alarming increase in heroin use across the country and an epidemic of drug overdose deaths".[201][175][202]
As a result, health care organizations and public health groups, such as Physicians for Responsible Opioid Prescribing, have called for decreases in the prescription of opioids.[201] In 2016, the Centers for Disease Control and Prevention (CDC) issued a new set of guidelines for the prescription of opioids "for chronic pain outside of active cancer treatment, palliative care, and end-of-life care".[203]
Society and culture
Definition
The term "opioid" originated in the 1950s.[204] It combines "opium" + "-oid" meaning "opiate-like" ("opiates" being morphine and similar drugs derived from opium). The first scientific publication to use it, in 1963, included a footnote stating, "In this paper, the term, 'opioid', is used in the sense originally proposed by George H. Acheson (personal communication) to refer to any chemical compound with morphine-like activities".[205] By the late 1960s, research found that opiate effects are mediated by activation of specific molecular receptors in the nervous system, which were termed "opioid receptors".[206] The definition of "opioid" was later refined to refer to substances that have morphine-like activities that are mediated by the activation of opioid receptors. One modern pharmacology textbook states: "the term opioid applies to all agonists and antagonists with morphine-like activity, and also the naturally occurring and synthetic opioid peptides".[207] Another pharmacology reference eliminates the morphine-like requirement: "Opioid, a more modern term, is used to designate all substances, both natural and synthetic, that bind to opioid receptors (including antagonists)".[2] Some sources define the term opioid to exclude opiates, and others use opiate comprehensively instead of opioid, but opioid used inclusively is considered modern, preferred and is in wide use.[14]
Efforts to reduce abuse in the US
In 2011, the Obama administration released a white paper describing the administration's plan to deal with the opioid crisis. The administration's concerns about addiction and accidental overdosing have been echoed by numerous other medical and government advisory groups around the world.[156][208][209][210]
As of 2015, prescription drug monitoring programs exist in every state, except for Missouri.[211] These programs allow pharmacists and prescribers to access patients’ prescription histories in order to identify suspicious use. However, a survey of US physicians published in 2015 found that only 53% of doctors used these programs, while 22% were not aware that the programs were available to them.[212] The Centers for Disease Control and Prevention was tasked with establishing and publishing a new guideline, and was heavily lobbied.[213] In 2016, the United States Centers for Disease Control and Prevention published its Guideline for Prescribing Opioids for Chronic Pain, recommending that opioids only be used when benefits for pain and function are expected to outweigh risks, and then used at the lowest effective dosage, with avoidance of concurrent opioid and benzodiazepine use whenever possible.[214] Research has also suggested that automated algorithms may be able to identify patients who need more intensive screening and/ or intervention for non-medical opioid use; however, there is no standard for determining non-medical opioid use, and few algorithms have yet to be applied to real world settings.[215]
On August 10, 2017, Donald Trump declared the opioid crisis a (non-FEMA) national public health emergency.[216]
Global shortages
Morphine and other poppy-based medicines have been identified by the World Health Organization as essential in the treatment of severe pain. As of 2002, seven countries (USA, UK, Italy, Australia, France, Spain and Japan) use 77% of the world's morphine supplies, leaving many emerging countries lacking in pain relief medication.[217] The current system of supply of raw poppy materials to make poppy-based medicines is regulated by the International Narcotics Control Board under the provision of the 1961 Single Convention on Narcotic Drugs. The amount of raw poppy materials that each country can demand annually based on these provisions must correspond to an estimate of the country's needs taken from the national consumption within the preceding two years. In many countries, underprescription of morphine is rampant because of the high prices and the lack of training in the prescription of poppy-based drugs. The World Health Organization is now working with administrations from various countries to train healthworkers and to develop national regulations regarding drug prescription to facilitate a greater prescription of poppy-based medicines.[218]
Another idea to increase morphine availability is proposed by the Senlis Council, who suggest, through their proposal for Afghan Morphine, that Afghanistan could provide cheap pain relief solutions to emerging countries as part of a second-tier system of supply that would complement the current INCB regulated system by maintaining the balance and closed system that it establishes while providing finished product morphine to those suffering from severe pain and unable to access poppy-based drugs under the current system.
Recreational use
Opioids can produce strong feelings of euphoria[219] and are frequently used recreationally. Traditionally associated with illicit opioids such as heroin, prescription opioids are misused recreationally.
Drug misuse and non-medical use include the use of drugs for reasons or at doses other than prescribed. Opioid misuse can also include providing medications to persons for whom it was not prescribed. Such diversion may be treated as crimes, punishable by imprisonment in many countries.[220][221] In 2014, almost 2 million Americans abused or were dependent on prescription opioids.[222]
Classification
There are a number of broad classes of opioids:[citation needed]
- Natural opiates: alkaloids contained in the resin of the opium poppy, primarily morphine, codeine, and thebaine, but not papaverine and noscapine which have a different mechanism of action; The following could be considered natural opiates: The leaves from Mitragyna speciosa (also known as kratom) contain a few naturally-occurring opioids, active via Mu- and Delta receptors. Salvinorin A, found naturally in the Salvia divinorum plant, is a kappa-opioid receptor agonist.[citation needed]
Esters of morphine opiates: slightly chemically altered but more natural than the semi-synthetics, as most are morphine prodrugs, diacetylmorphine (morphine diacetate; heroin), nicomorphine (morphine dinicotinate), dipropanoylmorphine (morphine dipropionate), desomorphine, acetylpropionylmorphine, dibenzoylmorphine, diacetyldihydromorphine;[223][224]
Semi-synthetic opioids: created from either the natural opiates or morphine esters, such as hydromorphone, hydrocodone, oxycodone, oxymorphone, ethylmorphine and buprenorphine;
Fully synthetic opioids: such as fentanyl, pethidine, levorphanol, methadone, tramadol, tapentadol, and dextropropoxyphene;
Endogenous opioid peptides, produced naturally in the body, such as endorphins, enkephalins, dynorphins, and endomorphins. Morphine, and some other opioids, which are produced in small amounts in the body, are included in this category.
Tramadol and tapentadol, which act as monoamine uptake inhibitors also act as mild and potent agonists (respectively) of the μ-opioid receptor.[225] Both drugs produce analgesia even when naloxone, an opioid antagonist, is administered.[226]
Some minor opium alkaloids and various substances with opioid action are also found elsewhere, including molecules present in kratom, Corydalis, and Salvia divinorum plants and some species of poppy aside from Papaver somniferum. There are also strains which produce copious amounts of thebaine, an important raw material for making many semi-synthetic and synthetic opioids. Of all of the more than 120 poppy species, only two produce morphine.
Amongst analgesics there are a small number of agents which act on the central nervous system but not on the opioid receptor system and therefore have none of the other (narcotic) qualities of opioids although they may produce euphoria by relieving pain—a euphoria that, because of the way it is produced, does not form the basis of habituation, physical dependence, or addiction. Foremost amongst these are nefopam, orphenadrine, and perhaps phenyltoloxamine or some other antihistamines. Tricyclic antidepressants have painkilling effect as well, but they're thought to do so by indirectly activating the endogenous opioid system. Paracetamol is predominantly a centrally acting analgesic (non-narcotic) which mediates its effect by action on descending serotoninergic (5-hydroxy triptaminergic) pathways, to increase 5-HT release (which inhibits release of pain mediators). It also decreases cyclo-oxygenase activity. It has recently been discovered that most or all of the therapeutic efficacy of paracetamol is due to a metabolite, AM404, which enhances the release of serotonin and inhibits the uptake of anandamide.[citation needed]
Other analgesics work peripherally (i.e., not on the brain or spinal cord). Research is starting to show that morphine and related drugs may indeed have peripheral effects as well, such as morphine gel working on burns. Recent investigations discovered opioid receptors on peripheral sensory neurons.[227] A significant fraction (up to 60%) of opioid analgesia can be mediated by such peripheral opioid receptors, particularly in inflammatory conditions such as arthritis, traumatic or surgical pain.[228] Inflammatory pain is also blunted by endogenous opioid peptides activating peripheral opioid receptors.[229]
It was discovered in 1953,[citation needed] that humans and some animals naturally produce minute amounts of morphine, codeine, and possibly some of their simpler derivatives like heroin and dihydromorphine, in addition to endogenous opioid peptides. Some bacteria are capable of producing some semi-synthetic opioids such as hydromorphone and hydrocodone when living in a solution containing morphine or codeine respectively.
Many of the alkaloids and other derivatives of the opium poppy are not opioids or narcotics; the best example is the smooth-muscle relaxant papaverine. Noscapine is a marginal case as it does have CNS effects but not necessarily similar to morphine, and it is probably in a category all its own.
Dextromethorphan (the stereoisomer of levomethorphan, a semi-synthetic opioid agonist) and its metabolite dextrorphan have no opioid analgesic effect at all despite their structural similarity to other opioids; instead they are potent NMDA antagonists and sigma 1 and 2-receptor agonists and are used in many over-the-counter cough suppressants.
Salvinorin A is a unique selective, powerful ĸ-opioid receptor agonist. It is not properly considered an opioid nevertheless, because:
- chemically, it is not an alkaloid; and
- it has no typical opioid properties: absolutely no anxiolytic or cough-suppressant effects. It is instead a powerful hallucinogen.
| Opioid peptides | Skeletal molecular images |
|---|---|
Adrenorphin |  |
Amidorphin |  |
Casomorphin | |
DADLE | |
DAMGO |  |
Dermorphin | |
Endomorphin |  |
Morphiceptin |  |
Nociceptin |  |
Octreotide |  |
Opiorphin |  |
TRIMU 5 |  |
Endogenous opioids
Opioid-peptides that are produced in the body include:
- Endorphins
- Enkephalins
- Dynorphins
- Endomorphins
β-endorphin is expressed in Pro-opiomelanocortin (POMC) cells in the arcuate nucleus, in the brainstem and in immune cells, and acts through μ-opioid receptors. β-endorphin has many effects, including on sexual behavior and appetite. β-endorphin is also secreted into the circulation from pituitary corticotropes and melanotropes. α-neo-endorphin is also expressed in POMC cells in the arcuate nucleus.
met-enkephalin is widely distributed in the CNS and in immune cells; [met]-enkephalin is a product of the proenkephalin gene, and acts through μ and δ-opioid receptors. leu-enkephalin, also a product of the proenkephalin gene, acts through δ-opioid receptors.
Dynorphin acts through κ-opioid receptors, and is widely distributed in the CNS, including in the spinal cord and hypothalamus, including in particular the arcuate nucleus and in both oxytocin and vasopressin neurons in the supraoptic nucleus.
Endomorphin acts through μ-opioid receptors, and is more potent than other endogenous opioids at these receptors.
Opium alkaloids and derivatives
Opium alkaloids
Phenanthrenes naturally occurring in (opium):
- Codeine
- Morphine
- Thebaine
Oripavine[230]
Preparations of mixed opium alkaloids, including papaveretum, are still occasionally used.
Esters of morphine
Diacetylmorphine (morphine diacetate; heroin)
Nicomorphine (morphine dinicotinate)
Dipropanoylmorphine (morphine dipropionate)- Diacetyldihydromorphine
- Acetylpropionylmorphine
- Desomorphine
- Methyldesorphine
- Dibenzoylmorphine
Ethers of morphine
- Dihydrocodeine
- Ethylmorphine
- Heterocodeine
Semi-synthetic alkaloid derivatives
- Buprenorphine
- Etorphine
- Hydrocodone
- Hydromorphone
- Oxycodone
- Oxymorphone
Synthetic opioids
Anilidopiperidines
- Fentanyl
- Alphamethylfentanyl
- Alfentanil
- Sufentanil
- Remifentanil
- Carfentanyl
- Ohmefentanyl
Phenylpiperidines
Pethidine (meperidine)- Ketobemidone
- MPPP
- Allylprodine
- Prodine
- PEPAP
- Promedol
Diphenylpropylamine derivatives
- Propoxyphene
- Dextropropoxyphene
- Dextromoramide
- Bezitramide
- Piritramide
- Methadone
- Dipipanone
Levomethadyl Acetate (LAAM)- Difenoxin
- Diphenoxylate
Loperamide (does cross the blood-brain barrier but is quickly pumped into the non-central nervous system by P-Glycoprotein. Mild opiate withdrawal in animal models exhibits this action after sustained and prolonged use including rhesus monkeys, mice, and rats.)
Benzomorphan derivatives
Dezocine—agonist/antagonist
Pentazocine—agonist/antagonist- Phenazocine
Oripavine derivatives
Buprenorphine—partial agonist- Dihydroetorphine
- Etorphine
Morphinan derivatives
Butorphanol—agonist/antagonist
Nalbuphine—agonist/antagonist- Levorphanol
- Levomethorphan
- Racemethorphan
Others
- Lefetamine
Menthol (Kappa-Opioid agonist)- Meptazinol
- Mitragynine
- Tilidine
- Tramadol
- Tapentadol
- Eluxadoline
- AP-237
- 7-Hydroxymitragynine
Allosteric modulators
Plain allosteric modulators do not belong to the opioids, instead they are classified as opioidergics.
Opioid antagonists
- Nalmefene
- Naloxone
- Naltrexone
Methylnaltrexone (Methylnaltrexone is only peripherally active as it does not cross the blood-brain barrier in sufficient quantities to be centrally active. As such, it can be considered the antithesis of loperamide.)
Naloxegol (Naloxegol is only peripherally active as it does not cross the blood-brain barrier in sufficient quantities to be centrally active. As such, it can be considered the antitheses of loperamide.)
Tables of opioids
Table of morphinan opioids
| Table of morphinan opioids: click to | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table of non-morphinan opioids
| Table of non-morphinan opioids: click to | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
See also
- Froehde reagent
- Opiate comparison
- Opioid epidemic
References
^ Ogura, Takahiro; Egan, Talmage D. (2013). "Chapter 15 – Opioid Agonists and Antagonists". Pharmacology and physiology for anesthesia : foundations and clinical application. Philadelphia, PA: Elsevier/Saunders. ISBN 978-1-4377-1679-5..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ ab Hemmings, Hugh C.; Egan, Talmage D. (2013). Pharmacology and Physiology for Anesthesia: Foundations and Clinical Application: Expert Consult - Online and Print. Elsevier Health Sciences. p. 253. ISBN 978-1437716795.Opiate is the older term classically used in pharmacology to mean a drug derived from opium. Opioid, a more modern term, is used to designate all substances, both natural and synthetic, that bind to opioid receptors (including antagonists).
^ ab Stromgaard, Kristian; Krogsgaard-Larsen, Povl; Madsen, Ulf (2009). Textbook of Drug Design and Discovery, Fourth Edition. CRC Press. ISBN 9781439882405.
^ Sterken, Joeri; Troubleyn, Joris; Gasthuys, Frank; Maes, Viviane; Diltoer, Mark; Verborgh, Christian (2004-10-01). "Intentional overdose of Large Animal Immobilon". European Journal of Emergency Medicine. 11 (5): 298–301. doi:10.1097/00063110-200410000-00013. ISSN 0969-9546. PMID 15359207.
^ Lembke, Anna (2016). Drug Dealer, MD: How Doctors Were Duped, Patients Got Hooked, and Why It's So Hard to Stop. Johns Hopkins University Press. ISBN 978-1421421407.
^ "FDA requires strong warnings for opioid analgesics, prescription opioid cough products, and benzodiazepine labeling related to serious risks and death from combined use". FDA. August 31, 2016. Retrieved 1 September 2016.
^ "Status and Trend Analysis of Illict [sic] Drug Markets". World Drug Report 2015 (PDF). Retrieved 26 June 2015.
^ "Report III: FDA Approved Medications for the Treatment of Opiate Dependence: Literature Reviews on Effectiveness & Cost- Effectiveness, Treatment Research Institute". Advancing Access to Addiction Medications: Implications for Opioid Addiction Treatment. p. 41.
^ Tetrault, Jeanette M.; Butner, Jenna L. (2015-09-03). "Non-Medical Prescription Opioid Use and Prescription Opioid Use Disorder: A Review". The Yale Journal of Biology and Medicine. 88 (3): 227–233. ISSN 0044-0086. PMC 4553642. PMID 26339205.
^ Tarabar, Asim F.; Nelson, Lewis S. (2003-04-01). "The resurgence and abuse of heroin by children in the United States". Current Opinion in Pediatrics. 15 (2): 210–215. doi:10.1097/00008480-200304000-00013. ISSN 1040-8703. PMID 12640281.
^ Gray, Eliza (2014-02-04). "Heroin Gains Popularity as Cheap Doses Flood the U.S". TIME.com. Retrieved 2016-02-12.
^ Maltoni, M. (2008-01-01). "Opioids, pain, and fear". Annals of Oncology. 19 (1): 5–7. doi:10.1093/annonc/mdm555. ISSN 0923-7534. PMID 18073220.[A] number of studies, however, have also reported inadequate pain control in 40%–70% of patients, resulting in the emergence of a new type of epidemiology, that of ‘failed pain control’, caused by a series of obstacles preventing adequate cancer pain management.... The cancer patient runs the risk of becoming an innocent victim of a war waged against opioid abuse and addiction if the norms regarding the two kinds of use (therapeutic or nontherapeutic) are not clearly distinct. Furthermore, health professionals may be worried about regulatory scrutiny and may opt not to use opioid therapy for this reason.
^ McCarberg, Bill H. (2011-03-01). "Pain management in primary care: strategies to mitigate opioid misuse, abuse, and diversion". Postgraduate Medicine. 123 (2): 119–130. doi:10.3810/pgm.2011.03.2270. ISSN 1941-9260. PMID 21474900.
^ abc Offermanns, Stefan (2008). Encyclopedia of Molecular Pharmacology. 1 (2 ed.). Springer Science & Business Media. p. 903. ISBN 9783540389163.In the strict sense, opiates are drugs derived from opium and include the natural products morphine, codeine, thebaine and many semi-synthetic congeners derived from them. In the wider sense, opiates are morphine-like drugs with non peptidic structures. The older term opiates is now more and more replaced by the term opioids which applies to any substance, whether endogenous or synthetic, peptidic or non-peptidic, that produces morphine-like effects through action on opioid receptors.
^ Freye, Enno (2008). "Part II. Mechanism of action of opioids and clinical effects". Opioids in Medicine: A Comprehensive Review on the Mode of Action and the Use of Analgesics in Different Clinical Pain States. Springer Science & Business Media. p. 85. ISBN 9781402059476.Opiate is a specific term that is used to describe drugs (natural and semi-synthetic) derived from the juice of the opium poppy. For example morphine is an opiate but methadone (a completely synthetic drug) is not. Opioid is a general term that includes naturally occurring, semi-synthetic, and synthetic drugs, which produce their effects by combining with opioid receptors and are competitively antagonized by nalaxone. In this context the term opioid refers to opioid agonists, opioid antagonists, opioid peptides, and opioid receptors.
^ ARNP, Pamela Davies MS; CNS, Yvonne D'Arcy MS, CRNP (2012-09-26). Compact Clinical Guide to Cancer Pain Management: An Evidence-Based Approach for Nurses. Springer Publishing Company. ISBN 9780826109743.
^ "21 U.S. Code § 802 - Definitions". LII / Legal Information Institute. Retrieved 2016-02-12.
^ "Definition of NARCOTIC". www.merriam-webster.com. Retrieved 2016-02-12.
^ Satoskar, R. S.; Rege, Nirmala; Bhandarkar, S. D. (2015). Pharmacology and Pharmacotherapeutics. Elsevier Health Sciences. ISBN 9788131243718.
^ Ebert, Michael H.; Kerns, Robert D. (2010). Behavioral and Psychopharmacologic Pain Management. Cambridge University Press. ISBN 9781139493543.
^ Moore, R. Andrew; Wiffen, Philip J.; Derry, Sheena; Maguire, Terry; Roy, Yvonne M.; Tyrrell, Laila (2015). "Non-prescription (OTC) oral analgesics for acute pain - an overview of Cochrane reviews". The Cochrane Database of Systematic Reviews. 11 (11): CD010794. doi:10.1002/14651858.CD010794.pub2. ISSN 1469-493X. PMID 26544675.
^ ab Fleisher, Gary R.; Ludwig, Stephen (2010). Textbook of Pediatric Emergency Medicine. Lippincott Williams & Wilkins. p. 61. ISBN 9781605471594.
^ Alexander GC, Kruszewski SP, Webster DW (2012). "Rethinking Opioid Prescribing to Protect Patient Safety and Public Health". JAMA. 308 (18): 1865–1866. doi:10.1001/jama.2012.14282. PMID 23150006.
^ Mohamadi, Amin; Chan, Jimmy J.; Lian, Jayson; Wright, Casey L.; Marin, Arden M.; Rodriguez, Edward K.; von Keudell, Arvind; Nazarian, Ara (2018-08-01). "Risk Factors and Pooled Rate of Prolonged Opioid Use Following Trauma or Surgery: A Systematic Review and Meta-(Regression) Analysis". The Journal of Bone and Joint Surgery. American Volume. 100 (15): 1332–1340. doi:10.2106/JBJS.17.01239. ISSN 1535-1386. PMID 30063596.
^ Franklin, G. M. (29 September 2014). "Opioids for chronic noncancer pain: A position paper of the American Academy of Neurology". Neurology. 83 (14): 1277–1284. doi:10.1212/WNL.0000000000000839. PMID 25267983.
^ ab Okie S (November 2010). "A flood of opioids, a rising tide of deaths". N. Engl. J. Med. 363 (21): 1981–5. doi:10.1056/NEJMp1011512. PMID 21083382.
Responses to Okie's perspective: "Opioids and deaths". N. Engl. J. Med. 364 (7): 686–7. February 2011. doi:10.1056/NEJMc1014490.
^ Dowell, D; Haegerich, TM; Chou, R (19 April 2016). "CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016". JAMA. 315 (15): 1624–45. doi:10.1001/jama.2016.1464. PMID 26977696.
^ McNicol E, Strassels SA, Goudas L, Lau J, Carr DB (2005). "NSAIDS or paracetamol, alone or combined with opioids, for cancer pain". Cochrane Database Syst Rev (1): CD005180. doi:10.1002/14651858.CD005180. PMID 15654708.
^ ab For information on the use and overuse of opioids to treat migraines, see American Academy of Neurology (February 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Academy of Neurology, retrieved 1 August 2013, which cites
Silberstein SD (2000). "Practice parameter: Evidence-based guidelines for migraine headache (an evidence-based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology". Neurology. 55 (6): 754–762. doi:10.1212/WNL.55.6.754. PMID 10993991.
Evers S, Afra J, Frese A, Goadsby PJ, Linde M, May A, Sándor PS (2009). "EFNS guideline on the drug treatment of migraine - revised report of an EFNS task force". European Journal of Neurology. 16 (9): 968–981. doi:10.1111/j.1468-1331.2009.02748.x. PMID 19708964.CS1 maint: Multiple names: authors list (link)
Institute for Clinical Systems Improvement (2011), Headache, Diagnosis and Treatment of, Institute for Clinical Systems Improvement
^ Painter JT, Crofford LJ (2013). "Chronic Opioid Use in Fibromyalgia Syndrome". Journal of Clinical Rheumatology. 19 (2): 72–77. doi:10.1097/RHU.0b013e3182863447. PMID 23364665.
^ McNicol ED, Midbari A, Eisenberg E (2013). "Opioids for neuropathic pain". Cochrane Database Syst Rev. 8 (8): CD006146. doi:10.1002/14651858.CD006146.pub2. PMID 23986501.
^ abc American Headache Society (September 2013), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Headache Society, archived from the original on 3 December 2013, retrieved 10 December 2013, which cites
Bigal ME, Lipton RB (2009). "Excessive opioid use and the development of chronic migraine". Pain. 142 (3): 179–182. doi:10.1016/j.pain.2009.01.013. PMID 19232469.
Bigal ME, Serrano D, Buse D, Scher A, Stewart WF, Lipton RB (2008). "Acute Migraine Medications and Evolution from Episodic to Chronic Migraine: A Longitudinal Population-Based Study". Headache: The Journal of Head and Face Pain. 48 (8): 1157–1168. doi:10.1111/j.1526-4610.2008.01217.x. PMID 18808500.
Scher AI, Stewart WF, Ricci JA, Lipton RB (2003). "Factors associated with the onset and remission of chronic daily headache in a population-based study". Pain. 106 (1–2): 81–89. doi:10.1016/S0304-3959(03)00293-8. PMID 14581114.
Katsarava Z, Schneeweiss S, Kurth T, Kroener U, Fritsche G, Eikermann A, Diener HC, Limmroth V (2004). "Incidence and predictors for chronicity of headache in patients with episodic migraine". Neurology. 62 (5): 788–790. doi:10.1212/01.WNL.0000113747.18760.D2. PMID 15007133.
^ Manchikanti L, Helm S 2nd, Fellows B, Janata JW, Pampati V, Grider JS, Boswell MV (Jul 2012). "Opioid epidemic in the United States". Pain Physician. 15 (3 Suppl): ES9–38. PMID 22786464.CS1 maint: Multiple names: authors list (link)
^ Chou, Roger; Ballantyne, Jane C.; Fanciullo, Gilbert J.; Fine, Perry G.; Miaskowski, Christine (2009). "Research Gaps on Use of Opioids for Chronic Noncancer Pain: Findings From a Review of the Evidence for an American Pain Society and American Academy of Pain Medicine Clinical Practice Guideline". The Journal of Pain. 10 (2): 147–159.e15. doi:10.1016/j.jpain.2008.10.007. PMID 19187891.
^ "PAIN". Painjournalonline.com. 2015-09-01. Retrieved 2016-01-07.
^ Kissin I (2015-09-28). "Long-term opioid treatment of chronic nonmalignant pain: unproven efficacy and neglected safety?". J Pain Res. 6: 513–29. doi:10.2147/JPR.S47182. PMC 3712997. PMID 23874119.
^ Dhalla, Irfan A.; Gomes, Tara; Mamdani, Muhammad M.; Juurlink, David N. (2012). "Opioids versus nonsteroidal anti-inflammatory drugs in noncancer pain". Canadian Family Physician. 58 (1): 30. ISSN 0008-350X. PMC 3264005. PMID 22267615.
^ Marret, E.; Beloeil, H.; Lejus, C. (2009). "[What are the benefits and risk of non-opioid analgesics combined with postoperative opioids?]". Annales Françaises d'Anesthésie et de Réanimation. 28 (3): e135–151. doi:10.1016/j.annfar.2009.01.006. ISSN 1769-6623. PMID 19304445.
^ Franceschi, F.; Iacomini, P.; Marsiliani, D.; Cordischi, C.; Antonini, E. Forte S.; Alesi, A.; Giacobelli, D.; Zuccalà, G. (2013). "Safety and efficacy of the combination acetaminophen-codeine in the treatment of pain of different origin" (pdf). European Review for Medical and Pharmacological Sciences. 17 (16): 2129–2135. ISSN 1128-3602. PMID 23893177.
^ ed, Kian Fan Chung ... (2008). Pharmacology and therapeutics of cough. Berlin: Springer. p. 248. ISBN 9783540798422.
^ Bolser DC, Davenport PW (February 2007). "Codeine and cough: an ineffective gold standard". Current Opinion in Allergy and Clinical Immunology. 7 (1): 32–6. doi:10.1097/ACI.0b013e3280115145. PMC 2921574. PMID 17218808.
^ ab Goldman, RD (Dec 2010). "Codeine for acute cough in children". Canadian Family Physician. 56 (12): 1293–4. PMC 3001921. PMID 21156892.
^ Paul, IM (Feb 2012). "Therapeutic options for acute cough due to upper respiratory infections in children". Lung. 190 (1): 41–4. doi:10.1007/s00408-011-9319-y. PMID 21892785.
^ Verlee, L; Verheij, TJ; Hopstaken, RM; Prins, JM; Salomé, PL; Bindels, PJ (2012). "[Summary of NHG practice guideline 'Acute cough']". Nederlands Tijdschrift voor Geneeskunde. 156: A4188. PMID 22917039.
^ Matthys, H.; Bleicher, B.; Bleicher, U. (1983). "Dextromethorphan and codeine: objective assessment of antitussive activity in patients with chronic cough". The Journal of International Medical Research. 11 (2): 92–100. doi:10.1177/030006058301100206. ISSN 0300-0605. PMID 6852361.
^ Van Amburgh JA. "Do Cough Remedies Work?". Medscape. Retrieved 10 April 2016.
^ Bolser, Donald C. (2010-02-01). "Pharmacologic Management of Cough". Otolaryngologic Clinics of North America. 43 (1): 147–155. doi:10.1016/j.otc.2009.11.008. ISSN 0030-6665. PMC 2827356. PMID 20172264.
^ Webster, Lynn R. (2015-10-01). "Opioid-Induced Constipation". Pain Medicine (Malden, Mass.). 16 Suppl 1: S16–21. doi:10.1111/pme.12911. ISSN 1526-4637. PMID 26461071.
^ "Press Announcements - FDA approves Movantik for opioid-induced constipation". www.fda.gov. Retrieved 2016-02-18.
^ Gallagher R (2011). "The use of opioids for dyspnea in advanced disease". Canadian Medical Association Journal. 183 (10): 1170. doi:10.1503/cmaj.110024. PMC 3134725. PMID 21482650.
^ Wiseman, R; Rowett, D; Allcroft, P; Abernethy, A; Currow, DC (Mar 2013). "Chronic refractory dyspnoea--evidence based management". Australian Family Physician. 42 (3): 137–40. PMID 23529525.
^ abcdefgh Furlan AD, Sandoval JA, Mailis-Gagnon A, Tunks E (2006). "Opioids for chronic noncancer pain: A meta-analysis of effectiveness and side effects". Canadian Medical Association Journal. 174 (11): 1589–1594. doi:10.1503/cmaj.051528. PMC 1459894. PMID 16717269.
^ Baumann S (2009). "A nursing approach to pain in older adults". Medsurg Nurs. 18 (2): 77–82, quiz 83. PMID 19489204.
^ Buckeridge D, Huang A, Hanley J, Kelome A, Reidel K, Verma A, Winslade N, Tamblyn R (September 2010). "Risk of injury associated with opioid use in older adults". J Am Geriatr Soc. 58 (9): 1664–70. doi:10.1111/j.1532-5415.2010.03015.x. PMID 20863326.
^ Schneider JP. Rational use of opioid analgesics in chronic musculoskeletal pain. J Musculoskel Med. 2010;27:142-148.
^ Wolff K (2002). "Characterization of methadone overdose: Clinical considerations and the scientific evidence". Therapeutic Drug Monitoring. 24 (4): 457–70. doi:10.1097/00007691-200208000-00001. PMID 12142628.
^ Teichtahl H, Wang D (2007). "Sleep-disordered breathing with chronic opioid use". Expert Opinion on Drug Safety. 6 (6): 641–9. doi:10.1517/14740338.6.6.641. PMID 17967153.
^ Center for Drug Evaluation and Research (23 August 2013). "Postmarket Drug Safety Information for Patients and Providers - Information for Healthcare Professionals Methadone Hydrochloride". U.S. Food and Drug Administration. Retrieved 24 February 2016.Methadone’s elimination half-life (8-59 hours) is longer than its duration of analgesic action (4-8 hours).
^ Stephens, Everett (23 November 2015). "Opioid Toxicity". Medscape. Retrieved 24 February 2016.The CDC reported that methadone contributed to 31.4% of opioid-related deaths in the United States from 1999-2010. Methadone also accounted for 39.8% of all single-drug opioid-related deaths. The overdose death rate associated with methadone was significantly higher than that associated with other opioid-related deaths among multidrug and single-drug deaths.
^ abcde Noble, M.; Treadwell, J. R.; Tregear, S. J.; Coates, V. H.; Wiffen, P. J.; Akafomo, C.; Schoelles, K. M. (2010). Noble, Meredith, ed. "Long-term opioid management for chronic noncancer pain". Cochrane Database of Systematic Reviews (1): CD006605. doi:10.1002/14651858.CD006605.pub2. PMID 20091598.
^ "Drug Overdose Deaths in the United States, 1999–2016" (PDF). CDC. Retrieved 23 December 2017.
^ abcde Overdose Death Rates. By National Institute on Drug Abuse (NIDA).
^ Fentanyl. Image 4 of 17. US DEA (Drug Enforcement Administration).
^ Pradhan; et al. (2010). "Ligand-directed trafficking of the δ-opioid receptor in vivo: two paths toward analgesic tolerance". J Neurosci. 30 (49): 16459–68. doi:10.1523/JNEUROSCI.3748-10.2010. PMC 3086517. PMID 21147985.
^ Kollars JP, Larson MD (March 2005). "Tolerance to miotic effects of opioids". Anesthesiology. 102 (3): 701. doi:10.1097/00000542-200503000-00047. PMID 15731628.
^ Santillán R, Maestre JM, Hurlé MA, Flórez J (Jul 1994). "Enhancement of opiate analgesia by nimodipine in cancer patients chronically treated with morphine: a preliminary report". Pain. 58 (1): 129–32. doi:10.1016/0304-3959(94)90192-9. PMID 7970835.
^ Santillán R1, Hurlé MA, Armijo JA, de los Mozos R, Flórez J. (May 1998). "Nimodipine-enhanced opiate analgesia in cancer patients requiring morphine dose escalation: a double-blind, placebo-controlled study". Pain. 76 (1–2): 17–26. doi:10.1016/S0304-3959(98)00019-0. PMID 9696455.CS1 maint: Multiple names: authors list (link)
^ Smith FL, Dombrowski DS, Dewey WL (Feb 1999). "Involvement of intracellular calcium in morphine tolerance in mice". Pharmacology Biochemistry and Behavior. 62 (2): 381–8. doi:10.1016/S0091-3057(98)00168-3. PMID 9972707.
^ McCarthy RJ, Kroin JS, Tuman KJ, Penn RD, Ivankovich AD (Apr 1998). "Antinociceptive potentiation and attenuation of tolerance by intrathecal co-infusion of magnesium sulfate and morphine in rats". Anesthesia and Analgesia. 86 (4): 830–6. doi:10.1097/00000539-199804000-00028. PMID 9539610.
^ Morrison AP1, Hunter JM, Halpern SH, Banerjee A. (May 2013). "Effect of intrathecal magnesium in the presence or absence of local anaesthetic with and without lipophilic opioids: a systematic review and meta-analysis". British Journal of Anaesthesia. 110 (5): 702–12. doi:10.1093/bja/aet064. PMID 23533255.CS1 maint: Multiple names: authors list (link)
^ Larson AA, Kovács KJ, Spartz AK (Nov 2000). "Intrathecal Zn2+ attenuates morphine antinociception and the development of acute tolerance". European Journal of Pharmacology. 407 (3): 267–72. doi:10.1016/S0014-2999(00)00715-9. PMID 11068022.
^ Wong CS, Cherng CH, Luk HN, Ho ST, Tung CS (February 1996). "Effects of NMDA receptor antagonists on inhibition of morphine tolerance in rats: binding at mu-opioid receptors". Eur. J. Pharmacol. 297 (1–2): 27–33. doi:10.1016/0014-2999(95)00728-8. PMID 8851162.
^ Malec D, Mandryk M, Fidecka S (Mar–Apr 2008). "Interaction of memantine and ketamine in morphine- and pentazocine-induced antinociception in mice" (PDF). Pharmacological Reports. 60 (2): 149–55. PMID 18443375. Retrieved 17 September 2011.
^ McCleane GJ (2003). "The cholecystokinin antagonist proglumide enhances the analgesic effect of dihydrocodeine". Clin J Pain. 19 (3): 200–1. doi:10.1097/00002508-200305000-00008. PMID 12792559.
^ Watkins LR, Kinscheck IB, Mayer DJ (Apr 1984). "Potentiation of opiate analgesia and apparent reversal of morphine tolerance by proglumide". Science. 224 (4647): 395–6. doi:10.1126/science.6546809. PMID 6546809.
^ Tang J, Chou J, Iadarola M, Yang HY, Costa E (Jun 1984). "Proglumide prevents and curtails acute tolerance to morphine in rats". Neuropharmacology. 23 (6): 715–8. doi:10.1016/0028-3908(84)90171-0. PMID 6462377.
^ Ledeboer A, Hutchinson MR, Watkins LR, Johnson KW (Jul 2007). "Ibudilast (AV-411). A new class therapeutic candidate for neuropathic pain and opioid withdrawal syndromes". Expert Opinion on Investigational Drugs. 16 (7): 935–50. doi:10.1517/13543784.16.7.935. PMID 17594181.
^ abcd Doyle, D.; Hanks, G.; Cherney, I.; et al., eds. (2004). Oxford Textbook of Palliative Medicine (3rd ed.). Oxford University Press. ISBN 978-0198566984.
^ Hermann D, Klages E, Welzel H, Mann K, Croissant B (June 2005). "Low efficacy of non-opioid drugs in opioid withdrawal symptoms". Addict Biol. 10 (2): 165–9. doi:10.1080/13556210500123514. PMID 16191669.
^ Brown, TK (March 2013). "Ibogaine in the treatment of substance dependence". Current Drug Abuse Reviews. 6 (1): 3–16. doi:10.2174/15672050113109990001. PMID 23627782.
^ Bannwarth B (10 September 2012). "Will abuse deterrent formulations of opioid analgesics be successful in their purpose?". Drugs. 72 (12): 1713–1723. doi:10.2165/11635860-000000000-00000. PMID 22931520.
^ Schneider JP, Matthews M, Jamison RN (24 Oct 2010). "Abuse-deterrent and tamper-resistant opioid formulations: what is their role in addressing prescription opioid abuse?". CNS Drugs. 24 (80): 805–810. doi:10.2165/11584260-000000000-00000. PMID 20839893.
^ Xu Y, Johnson A (2013). "Opioid Therapy Pharmacogenomics for Noncancer Pain: Efficacy, Adverse Events, and Costs". Pain Research and Treatment. 2013: 1–8. doi:10.1155/2013/943014. PMC 3791560. PMID 24167729.
^ Brush DE (2012). "Complications of Long-Term Opioid Therapy for Management of Chronic Pain: The Paradox of Opioid-Induced Hyperalgesia". Journal of Medical Toxicology. 8 (4): 387–392. doi:10.1007/s13181-012-0260-0. PMC 3550256. PMID 22983894.
^ Malik, Zubair; Baik, Daniel; Schey, Ron (2015). "The role of cannabinoids in regulation of nausea and vomiting, and visceral pain". Current Gastroenterology Reports. 17 (2): 429. doi:10.1007/s11894-015-0429-1. ISSN 1534-312X. PMID 25715910.
^ Abrams, D. I.; Guzman, M. (2015). "Cannabis in cancer care". Clinical Pharmacology and Therapeutics. 97 (6): 575–586. doi:10.1002/cpt.108. ISSN 1532-6535. PMID 25777363.
^ "UCSF Study Finds Medical Marijuana Could Help Patients Reduce Pain with Opiates". UC San Francisco. Retrieved 2016-03-04.
^ Abrams, D. I.; Couey, P.; Shade, S. B.; Kelly, M. E.; Benowitz, N. L. (2011). "Cannabinoid-opioid interaction in chronic pain". Clinical Pharmacology and Therapeutics. 90 (6): 844–851. doi:10.1038/clpt.2011.188. ISSN 1532-6535. PMID 22048225.
^ Reissig, James E.; Rybarczyk, Amy M. (2005). "Pharmacologic treatment of opioid-induced sedation in chronic pain". The Annals of Pharmacotherapy. 39 (4): 727–731. doi:10.1345/aph.1E309. ISSN 1060-0280. PMID 15755795.
^ Corey, P. J.; Heck, A. M.; Weathermon, R. A. (1999). "Amphetamines to counteract opioid-induced sedation". The Annals of Pharmacotherapy. 33 (12): 1362–1366. doi:10.1345/aph.19024. ISSN 1060-0280. PMID 10630837.
^ Canadian Agency for Drugs and Technologies in Health (Jun 26, 2014). "Dioctyl Sulfosuccinate or Docusate (Calcium or Sodium) for the Prevention or Management of Constipation: A Review of the Clinical Effectiveness". PMID 25520993.
^ McCarberg BH (2013). "Overview and Treatment of Opioid-Induced Constipation". Postgraduate Medicine. 125 (4): 7–17. doi:10.3810/pgm.2013.07.2651. PMID 23782897.
^ Schwarzer, A; Nauck, F; Klaschik, E (June 2005). "[Strong opioids and constipation]". Schmerz (Berlin, Germany). 19 (3): 214–9. doi:10.1007/s00482-004-0325-3. PMID 15004747.
^ Dorn, Spencer; Lembo, Anthony; Cremonini, Filippo (10 September 2014). "Opioid-Induced Bowel Dysfunction: Epidemiology, Pathophysiology, Diagnosis, and Initial Therapeutic Approach". The American Journal of Gastroenterology Supplements. 2 (1): 31–37. doi:10.1038/ajgsup.2014.7. PMID 25207610.
^ abcde Kumar, Lalit; Barker, Chris; Emmanuel, Anton (2014). "Opioid-Induced Constipation: Pathophysiology, Clinical Consequences, and Management". Gastroenterology Research and Practice. 2014: 1–6. doi:10.1155/2014/141737. ISSN 1687-6121. PMC 4027019. PMID 24883055.
^ ab Patrick Craig Alguire; American College of Physicians; Clerkship Directors in Internal Medicine (2009). Internal Medicine Essentials for Clerkship Students 2. ACP Press. pp. 272–. ISBN 978-1-934465-13-4.
^ abc Jennifer A. Elliott; Howard S. Smith (19 April 2016). Handbook of Acute Pain Management. CRC Press. pp. 89–. ISBN 978-1-4665-9635-1.
^ Poulsen, J. L.; Brock, C.; Olesen, A. E.; Nilsson, M.; Drewes, A. M. (2015). "Evolving paradigms in the treatment of opioid-induced bowel dysfunction". Therapeutic Advances in Gastroenterology. 8 (6): 360–372. doi:10.1177/1756283X15589526. ISSN 1756-283X. PMC 4622283. PMID 26557892.
^ Davis, MD, FCCP, FAAHPM, Mellar P.; Goforth, MD, Harold W. (2016). "Oxycodone with an opioid receptor antagonist: A review". Journal of Opioid Management. 12 (1): 67–85. doi:10.5055/jom.2016.0313. ISSN 1551-7489. PMID 26908305.CS1 maint: Multiple names: authors list (link)
^ McNicol ED, Boyce D, Schumann R, Carr DB (2008). McNicol, Ewan D, ed. "Mu-opioid antagonists for opioid-induced bowel dysfunction". Cochrane Database Syst Rev (2): CD006332. doi:10.1002/14651858.CD006332.pub2. PMID 18425947.CS1 maint: Multiple names: authors list (link)
^ Yost CS (2006). "A new look at the respiratory stimulant doxapram". CNS Drug Rev. 12 (3–4): 236–49. doi:10.1111/j.1527-3458.2006.00236.x. PMID 17227289.
^ Tan ZM, Liu JH, Dong T, Li JX (August 2006). "[Clinical observation of target-controlled remifentanil infusion combined with propofol and doxapram in painless artificial abortion]". Nan Fang Yi Ke da Xue Xue Bao. 26 (8): 1206–8. PMID 16939923.
^ Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW (2003). "5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia". Science. 301 (5630): 226–9. doi:10.1126/science.1084674. PMID 12855812.
^ Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D (August 2007). "5-Hydroxytryptamine 1A/7 and 4alpha receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function". Hypertension. 50 (2): 368–76. doi:10.1161/HYPERTENSIONAHA.107.091033. PMID 17576856.
^ Ren J, Poon BY, Tang Y, Funk GD, Greer JJ (December 2006). "Ampakines alleviate respiratory depression in rats". Am. J. Respir. Crit. Care Med. 174 (12): 1384–91. doi:10.1164/rccm.200606-778OC. PMID 16973981.
^ Wilson GR, Reisfield GM (2003). "Morphine hyperalgesia: a case report". Am J Hosp Palliat Care. 20 (6): 459–61. doi:10.1177/104990910302000608. PMID 14649563.
^ Vella-Brincat J, Macleod AD (2007). "Adverse effects of opioids on the central nervous systems of palliative care patients". J Pain Palliat Care Pharmacother. 21 (1): 15–25. doi:10.1080/J354v21n01_05. PMID 17430825.
^ Mercadante S, Arcuri E (2005). "Hyperalgesia and opioid switching". Am J Hosp Palliat Care. 22 (4): 291–4. doi:10.1177/104990910502200411. PMID 16082916.
^ Fine PG (2004). "Opioid insights:opioid-induced hyperalgesia and opioid rotation". J Pain Palliat Care Pharmacother. 18 (3): 75–9. doi:10.1080/J354v18n03_08. PMID 15364634.
^ Lee, Marion; Silverman, Sanford M.; Hansen, Hans; Patel, Vikram B.; Manchikanti, Laxmaiah (2011). "A comprehensive review of opioid-induced hyperalgesia". Pain Physician. 14 (2): 145–61. ISSN 2150-1149. PMID 21412369.
^ Tompkins, D. Andrew; Campbell, Claudia M. (2011). "Opioid-Induced Hyperalgesia: Clinically Relevant or Extraneous Research Phenomenon?" (PDF). Current Pain and Headache Reports. 15 (2): 129–136. doi:10.1007/s11916-010-0171-1. ISSN 1531-3433. PMC 3165032. PMID 21225380.
^ Díaz JL, Zamanillo D, Corbera J, Baeyens JM, Maldonado R, Pericàs MA, Vela JM, Torrens A (September 2009). "Selective sigma-1 (sigma1) receptor antagonists: emerging target for the treatment of neuropathic pain". Central Nervous System Agents in Medicinal Chemistry. 9 (3): 172–83. doi:10.2174/1871524910909030172. PMID 20021351.
^ Mitra S (2008). "Opioid-induced hyperalgesia: pathophysiology and clinical implications". Journal of Opioid Management. 4 (3): 123–30. PMID 18717507.
^ Baron R (2009). "Neuropathic Pain: A Clinical Perspective". Sensory Nerves. Handbook of Experimental Pharmacology. 194. pp. 3–30. doi:10.1007/978-3-540-79090-7_1. ISBN 978-3-540-79089-1. PMID 19655103.
^ Candiotti KA, Gitlin MC (July 2010). "Review of the effect of opioid-related side effects on the undertreatment of moderate to severe chronic non-cancer pain: tapentadol, a step toward a solution?". Current Medical Research and Opinion. 26 (7): 1677–84. doi:10.1185/03007995.2010.483941. PMID 20465361.
^ Brennan MJ (March 2013). "The effect of opioid therapy on endocrine function". Am. J. Med. 126 (3 Suppl 1): S12–8. doi:10.1016/j.amjmed.2012.12.001. PMID 23414717.
^ Colameco S, Coren JS (January 2009). "Opioid-induced endocrinopathy". J Am Osteopath Assoc. 109 (1): 20–5. PMID 19193821.
^ Smith HS, Elliott JA (July 2012). "Opioid-induced androgen deficiency (OPIAD)". Pain Physician. 15 (3 Suppl): ES145–56. PMID 22786453.
^ Brede E, Mayer TG, Gatchel RJ (2012). "Prediction of failure to retain work 1 year after interdisciplinary functional restoration in occupational injuries". Arch Phys Med Rehabil. 93 (2): 268–74. doi:10.1016/j.apmr.2011.08.029. PMID 22289236.
^ Volinn E, Fargo JD, Fine PG (2009). "Opioid therapy for nonspecific low back pain and the outcome of chronic work loss". Pain. 142 (3): 194–201. doi:10.1016/j.pain.2008.12.017. PMID 19181448.
^ abc American College of Occupational and Environmental Medicine (February 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American College of Occupational and Environmental Medicine, retrieved 24 February 2014, which cites
Weiss, MS; Bowden, K; Branco, F; et al. (2011). "Opioids Guideline". In Kurt T. Hegmann. Occupational medicine practice guidelines : evaluation and management of common health problems and functional recovery in workers (online March 2014) (3rd ed.). Elk Grove Village, IL: American College of Occupational and Environmental Medicine. p. 11. ISBN 978-0615452272.
^ Cherubino P, Sarzi-Puttini P, Zuccaro SM, Labianca R (2012). "The management of chronic pain in important patient subgroups". Clin Drug Investig. 32 Suppl 1: 35–44. doi:10.2165/11630060-000000000-00000. PMID 23389874.
^ White KT, Dillingham TR, González-Fernández M, Rothfield L (2009). "Opiates for chronic nonmalignant pain syndromes: can appropriate candidates be identified for outpatient clinic management?". Am J Phys Med Rehabil. 88 (12): 995–1001. doi:10.1097/PHM.0b013e3181bc006e. PMID 19789432.
^ Kaye AM, Kaye AD, Lofton EC (2013). "Basic Concepts in Opioid Prescribing and Current Concepts of Opioid-Mediated Effects on Driving". The Ochsner Journal. 13 (4): 525–532. PMC 3865831. PMID 24358001.
^ Orriols L, Delorme B, Gadegbeku B, Tricotel A, Contrand B, Laumon B, Salmi LR, Lagarde E (2010). Pirmohamed, Munir, ed. "Prescription medicines and the risk of road traffic crashes: a French registry-based study". PLoS Med. 7 (11): e1000366. doi:10.1371/journal.pmed.1000366. PMC 2981588. PMID 21125020.CS1 maint: Multiple names: authors list (link)
^ Miller M, Stürmer T, Azrael D, Levin R, Solomon DH (2011). "Opioid analgesics and the risk of fractures in older adults with arthritis". J Am Geriatr Soc. 59 (3): 430–8. doi:10.1111/j.1532-5415.2011.03318.x. PMC 3371661. PMID 21391934.
^ abc Gudin JA, Mogali S, Jones JD, Comer SD (2013). "Risks, Management, and Monitoring of Combination Opioid, Benzodiazepines, and/or Alcohol Use". Postgraduate Medicine. 125 (4): 115–130. doi:10.3810/pgm.2013.07.2684. PMC 4057040. PMID 23933900.
^ Stuth EA, Stucke AG, Zuperku EJ (2012). Effects of Anesthetics, Sedatives, and Opioids on Ventilatory Control. Comprehensive Physiology. 2. pp. 2281–2367. doi:10.1002/cphy.c100061. ISBN 9780470650714. PMID 23720250.
^ Gowing, Linda; Ali, Robert; White, Jason M. (2017). "Opioid antagonists with minimal sedation for opioid withdrawal". The Cochrane Database of Systematic Reviews. 5: CD002021. doi:10.1002/14651858.CD002021.pub4. ISSN 1469-493X. PMID 28553701.
^ Miller, Ronald D. (2010). Miller's Anesthesia (7th ed.). Elsevier Health Sciences. ISBN 978-0-443-06959-8.
^ Morgan, G. Edward; Mikhail, Maged S.; Murray, Michael J. (2006). Clinical Anesthesiology (4th ed.). McGraw Hill. ISBN 978-0-07-110515-6.
^ Chestnut, David H.; Wong, Cynthia A.; Tsen, Lawrence C.; Kee, Warwick D. Ngan; Beilin, Yaakov; Mhyre, Jill (2014). Chestnut's Obstetric Anesthesia: Principles and Practice. Elsevier Health Sciences. p. 468. ISBN 9780323113748.The lipid solubility of hydromorphone lies between morphine and fentanyl, but is closer to that of morphine.
^ Le Naour M, Lunzer MM, Powers MD, et al. (2014). "Putative Kappa Opioid Heteromers As Targets for Developing Analgesics Free of Adverse Effects". J. Med. Chem. 57 (15): 6383–92. doi:10.1021/jm500159d. PMC 4136663. PMID 24978316.
^ DeWire SM; et al. (2013). "A G protein-biased ligand at the μ-opioid receptor is potently analgesic with reduced gastrointestinal and respiratory dysfunction compared with morphine". J Pharmacol Exp Ther. 344 (3): 708–17. doi:10.1124/jpet.112.201616. PMID 23300227.
^ abc Takayama H, Ishikawa H, Kurihara M, Kitajima M, Aimi N, Ponglux D, Koyama F, Matsumoto K, Moriyama T, Yamamoto LT, Watanabe K, Murayama T, Horie S (2002). "Studies on the synthesis and opioid agonistic activities of mitragynine-related indole alkaloids: discovery of opioid agonists structurally different from other opioid ligands". J. Med. Chem. 45 (9): 1949–56. doi:10.1021/jm010576e. PMID 11960505.
^ abcdefghijklmnopqrstuvwxyzaaabacadaeafagahaiajakalaman Raynor K, Kong H, Chen Y, Yasuda K, Yu L, Bell GI, Reisine T (1994). "Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors". Mol. Pharmacol. 45 (2): 330–4. PMID 8114680.
^ abcdefg Filizola M, Villar HO, Loew GH (January 2001). "Molecular determinants of non-specific recognition of delta, mu, and kappa opioid receptors". Bioorg. Med. Chem. 9 (1): 69–76. doi:10.1016/S0968-0896(00)00223-6. PMID 11197347.
^ abcdefgh Tam SW (1985). "(+)-[3H]SKF 10,047, (+)-[3H]ethylketocyclazocine, mu, kappa, delta and phencyclidine binding sites in guinea pig brain membranes". Eur. J. Pharmacol. 109 (1): 33–41. doi:10.1016/0014-2999(85)90536-9. PMID 2986989.
^ abcdefghi Corbett, A. D.; Paterson, S. J.; Kosterlitz, H. W. (1993). Opioids. Handbook of Experimental Pharmacology. 104 / 1. pp. 645–679. doi:10.1007/978-3-642-77460-7_26. ISBN 978-3-642-77462-1. ISSN 0171-2004.
^ abcdefghijklmnopqrs Codd EE, Shank RP, Schupsky JJ, Raffa RB (1995). "Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception". J. Pharmacol. Exp. Ther. 274 (3): 1263–70. PMID 7562497.
^ Frink MC, Hennies HH, Englberger W, Haurand M, Wilffert B (1996). "Influence of tramadol on neurotransmitter systems of the rat brain". Arzneimittelforschung. 46 (11): 1029–36. PMID 8955860.
^ Potschka H, Friderichs E, Löscher W (2000). "Anticonvulsant and proconvulsant effects of tramadol, its enantiomers and its M1 metabolite in the rat kindling model of epilepsy". Br. J. Pharmacol. 131 (2): 203–12. doi:10.1038/sj.bjp.0703562. PMC 1572317. PMID 10991912.
^ Katsumata S, Minami M, Nakagawa T, Iwamura T, Satoh M (1995). "Pharmacological study of dihydroetorphine in cloned mu-, delta- and kappa-opioid receptors". Eur. J. Pharmacol. 291 (3): 367–73. doi:10.1016/0922-4106(95)90078-0. PMID 8719422.
^ Bart G, Schluger JH, Borg L, Ho A, Bidlack JM, Kreek MJ (2005). "Nalmefene induced elevation in serum prolactin in normal human volunteers: partial kappa opioid agonist activity?". Neuropsychopharmacology. 30 (12): 2254–62. doi:10.1038/sj.npp.1300811. PMID 15988468.
^ Wentland MP, Lou R, Lu Q, Bu Y, VanAlstine MA, Cohen DJ, Bidlack JM (2009). "Syntheses and opioid receptor binding properties of carboxamido-substituted opioids". Bioorg. Med. Chem. Lett. 19 (1): 203–8. doi:10.1016/j.bmcl.2008.10.134. PMID 19027293.
^ ab Gharagozlou P, Demirci H, David Clark J, Lameh J (2003). "Activity of opioid ligands in cells expressing cloned mu opioid receptors". BMC Pharmacol. 3: 1. doi:10.1186/1471-2210-3-1. PMC 140036. PMID 12513698.
Gharagozlou P, Demirci H, Clark JD, Lameh J (2002). "Activation profiles of opioid ligands in HEK cells expressing delta opioid receptors". BMC Neurosci. 3: 19. PMC 137588. PMID 12437765.
Gharagozlou P, Hashemi E, DeLorey TM, Clark JD, Lameh J (2006). "Pharmacological profiles of opioid ligands at kappa opioid receptors". BMC Pharmacol. 6: 3. doi:10.1186/1471-2210-6-3. PMC 1403760. PMID 16433932.
^ Roth BL, Baner K, Westkaemper R, Siebert D, Rice KC, Steinberg S, Ernsberger P, Rothman RB (2002). "Salvinorin A: a potent naturally occurring nonnitrogenous kappa opioid selective agonist". Proc. Natl. Acad. Sci. U.S.A. 99 (18): 11934–9. doi:10.1073/pnas.182234399. PMC 129372. PMID 12192085.
^ Wentland MP, Lou R, Lu Q, Bu Y, Denhardt C, Jin J, Ganorkar R, VanAlstine MA, Guo C, Cohen DJ, Bidlack JM (2009). "Syntheses of novel high affinity ligands for opioid receptors". Bioorg. Med. Chem. Lett. 19 (8): 2289–94. doi:10.1016/j.bmcl.2009.02.078. PMC 2791460. PMID 19282177.
^ Gassaway MM, Rives ML, Kruegel AC, Javitch JA, Sames D (2014). "The atypical antidepressant and neurorestorative agent tianeptine is a μ-opioid receptor agonist". Transl Psychiatry. 4 (7): e411. doi:10.1038/tp.2014.30. PMC 4119213. PMID 25026323.
^ "Annual prevalence of use of drugs, by region and globally, 2016". World Drug Report 2018. United Nations Office on Drugs and Crime. 2018. Retrieved 7 July 2018.
^ Volkow, Nora D. "America's Addiction to Opioids: Heroin and Prescription Drug Abuse". DrugAbuse.GOV. National Institute on Drug Abuse. Retrieved April 30, 2017.
^ "Narcotic Drugs Stupéfiants Estupefacientes" (PDF). INTERNATIONAL NARCOTICS CONTROL BOARD. 2012. Retrieved March 6, 2017.
^ "Opioid Consumption Data | Pain & Policy Studies Group". Painpolicy.wisc.edu. Retrieved 2016-01-07.
^ Dell CA, Roberts G, Kilty J, Taylor K, Daschuk M, Hopkins C, Dell D (2012). "Researching Prescription Drug Misuse among First Nations in Canada: Starting from a Health Promotion Framework". Subst Abuse. 6: 23–31. doi:10.4137/SART.S9247. PMC 3411531. PMID 22879752.
^ "Socially disadvantaged Ontarians being prescribed opioids on an ongoing basis and at doses that far exceed Canadian guidelines". Ices.on.ca. 2011-01-25. Retrieved 2016-01-07.
^ ab "Avoiding Abuse, Achieving a Balance: Tackling the Opioid Public Health Crisis" (PDF). College of Physicians and Surgeons of Ontario. 8 September 2010. Archived from the original (PDF) on 7 June 2016. Retrieved 6 March 2017.
^ Manglik, Aashish; Kruse, Andrew C.; Kobilka, Tong Sun; Thian, Foon Sun; Mathiesen, Jesper M.; Sunahara, Roger K.; Pardo, Leonardo; Weis, William I.; Kobilka, Brian K. (2012-03-21). "Crystal structure of the μ-opioid receptor bound to a morphinan antagonist". Nature. 485 (7398): 321–326. doi:10.1038/nature10954. ISSN 0028-0836. PMC 3523197. PMID 22437502.Opium is one of the world’s oldest drugs, and its derivatives morphine and codeine are among the most used clinical drugs to relieve severe pain.
^ Colledge, Sue; Conolly, James (August 10, 2007). The Origins and Spread of Domestic Plants in Southwest Asia and Europe. Walnut Creek, CA: Left Coast Press. pp. 179–181. ISBN 978-1598749885. Retrieved 28 August 2018.
^ Kunzig, Robert; Tzar, Jennifer (November 1, 2002). "La Marmotta". Discover. Retrieved 28 August 2018.
^ Chevalier, Alexandre; Marinova, Elena; Pena-Chocarro, Leonor (April 1, 2014). Plants and People: Choices and Diversity through Time. Oxbow Books. pp. 97–99. ISBN 978-1842175149. Retrieved 28 August 2018.
^ Kritikos, P.G.; Papadaki, S.P. (1967). "The history of the poppy and of opium and their expansion in antiquity in the eastern Mediterranean area". Bulletin on Narcotics. XIX (4). Retrieved 23 August 2018.
^ Sonnedecker, Glenn (1962). "Emergence of the Concept of Opiate Addiction". Journal Mondial de Pharmacie. 3: 275–290.
^ abcdef Brownstein, M J (1993). "A brief history of opiates, opioid peptides, and opioid receptors" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 90 (12): 5391–5392. doi:10.1073/pnas.90.12.5391. ISSN 0027-8424. PMC 46725. PMID 8390660.
^ abcd Duarte, Danilo Freire (February 2005). "Uma breve história do ópio e dos opióides". Revista Brasileira de Anestesiologia. 55 (1). doi:10.1590/S0034-70942005000100015. Retrieved 23 August 2018.
^ Rosso, A. M. (2010). "Poppy and Opium in Ancient Times: Remedy or Narcotic?". Biomedicine International. 1: 81–87. CiteSeerX 10.1.1.846.221.
^ Astyrakaki, Elisabeth; Papaioannou, Alexandra; Askitopoulou, Helen (January 2010). "References to Anesthesia, Pain, and Analgesia in the Hippocratic Collection". Anesthesia & Analgesia. 110 (1): 188–194. doi:10.1213/ane.0b013e3181b188c2. PMID 19861359.
^ Türe, H.; Türe, U.; Gögüs, F.Y.; Valavanis, A.; Yasargil, M.G. (September 2006). "987 THE ART OF ALLEVIATING PAIN IN GREEK MYTHOLOGY". European Journal of Pain (Abstracts of Pain in Europe V 5th Congress of the European Federation of IASP Chapters (EFIC)). 10 (S1): S255b–S255. doi:10.1016/S1090-3801(06)60990-7.'Sedare dolorem opus divinum est – an old Latin inscription – means “alleviating pain is the work of the divine”. This inscription is often attributed to either Hippocrates of Kos or Galen of Pergamum, but it is most likely an anonymous proverb'
^ Osbaldeston, Tess Anne (2000). Dioscorides (translation). Johannesburg, South Africa: Ibidis Press. pp. 607–611. ISBN 978-0-620-23435-1.
^ Heydari, M; Hashempur, MH; Zargaran, A (2013). "Medicinal aspects of opium as described in Avicenna's Canon of Medicine". Acta Medico-historica Adriatica : AMHA. 11 (1): 101–12. PMID 23883087. Retrieved 28 August 2018.
^ abc Asthana, S. N. (1954). "The Cultivation of the Opium Poppy in India". Bulletin on Narcotics (3). Retrieved 5 September 2018.
^ abc Sigerist, H. E. (1941). "Laudanum in the Works of Paracelsus" (PDF). Bull. Hist. Med. 9: 530–544. Retrieved 5 September 2018.
^ abcde Hamilton, Gillian R.; Baskett, Thomas F. (April 2000). "In the arms of morpheus: the development of morphine for postoperative pain relief". Canadian Journal of Anesthesia/Journal Canadien d'Anesthésie. 47 (4): 367–374. doi:10.1007/BF03020955. PMID 10764185.
^ ab Farooqui, Amar (December 2016). "The Global Career of Indian Opium and Local Destinies" (PDF). Almanack (14): 52–73. doi:10.1590/2236-463320161404. Retrieved 5 September 2018.
^ ab Deming, Sarah (2011). "The Economic Importance of Indian Opium and Trade with China on Britain's Economy, 1843–1890" (PDF). Economic Working Papers. 25 (Spring). Retrieved 5 September 2018.
^ abcdefghijk Rinde, Meir (2018). "Opioids' Devastating Return". Distillations. 4 (2): 12–23. Retrieved August 23, 2018.
^ Mills, James H. (2016). Review of 'Opium and Empire in Southeast Asia: Regulating Consumption in British Burma'. Reviews in History. Cambridge imperial and post-colonial studies series. Palgrave Macmillan. doi:10.14296/RiH/2014/2010. ISBN 9780230296466. Retrieved 5 September 2018.
^ Krishnamurti, Chandrasekhar; Rao, SSCChakra (2016). "The isolation of morphine by Serturner". Indian Journal of Anaesthesia. 60 (11): 861–862. doi:10.4103/0019-5049.193696. PMC 5125194. PMID 27942064.
^ Courtwright, David T. (2009). Forces of habit drugs and the making of the modern world (1 ed.). Cambridge, Mass.: Harvard University Press. pp. 36–37. ISBN 9780674029903.
^ Atanasov, AG; Waltenberger, B; Pferschy-Wenzig, EM; Linder, T; Wawrosch, C; Uhrin, P; Temml, V; Wang, L; Schwaiger, S; Heiss, EH; Rollinger, JM; Schuster, D; Breuss, JM; Bochkov, V; Mihovilovic, MD; Kopp, B; Bauer, R; Dirsch, VM; Stuppner, H (December 2015). "Discovery and resupply of pharmacologically active plant-derived natural products: A review". Biotechnology Advances. 33 (8): 1582–1614. doi:10.1016/j.biotechadv.2015.08.001. PMC 4748402. PMID 26281720. Retrieved 6 September 2018.
^ Kotwal, Atul (March 2005). "Innovation, diffusion and safety of a medical technology: a review of the literature on injection practices". Social Science & Medicine. 60 (5): 1133–1147. doi:10.1016/j.socscimed.2004.06.044. PMID 15589680. Retrieved 6 September 2018.
^ Clayton J. Mosher (2013). Drugs and Drug Policy: The Control of Consciousness Alteration. SAGE Publications. p. 123. ISBN 9781483321882.
^ Fisher, Gary L. (2009). Encyclopedia of substance abuse prevention, treatment, & recovery. Los Angeles: SAGE. p. 564. ISBN 9781452266015.
^ ab Trickey, Erick (January 4, 2018). "Inside the Story of America's 19th-Century Opiate Addiction". Smithsonian. Retrieved 6 September 2018.
^ Schechter, Neil L. (1993). Pain in Infants, Children, and Adolescents. Williams & Wilkins. ISBN 9780683075885. Retrieved 6 September 2018.
^ Hicks, Robert D. (2011). "Frontline Pharmacies". Chemical Heritage Magazine. Chemical Heritage Foundation. Retrieved October 29, 2018.
^ ab Booth, Martin (1999-06-12). Opium : a history (1st U.S. ed.). St. Martin's Press. ISBN 978-0312206673.
^ Wisniak, Jaime (March 2013). "Pierre-Jean Robiquet" (PDF). Educación Química. 24: 139–149. doi:10.1016/S0187-893X(13)72507-2. Retrieved 30 October 2018.
^ Filan, Kenaz (2011). The power of the poppy : harnessing nature's most dangerous plant ally. Park Street Press. p. 69. ISBN 9781594773990. Retrieved 30 October 2018.
^ "Felix Hoffmann". Science History Institute. Retrieved 21 March 2018.
^ Cooper, Raymond; Deakin, Jeffrey John (Feb 22, 2016). Botanical miracles : chemistry of plants that changed the world. CRC Press. p. 137. ISBN 9781498704281.
^ Sneader, Walter (November 1998). "The discovery of heroin" (PDF). The Lancet. 352 (9141): 1697–1699. doi:10.1016/S0140-6736(98)07115-3. Retrieved 12 September 2018.Bayer registered the name heroin in June, 1898.
^ abcde Newton, David E. (2016). Youth substance abuse : a reference handbook. Santa Barbara, CA: ABC-CLIO. pp. 41–60. ISBN 9781440839832. Retrieved 30 October 2018.
^ Crow, James Mitchell (3 January 2017). "Addicted to the cure". Chemistry World. Retrieved 30 October 2018.
^ Methadone Matters: Evolving Community Methadone Treatment of Opiate Addiction. CRC Press. 2003. p. 13. ISBN 9780203633090. Archived from the original on 2015-12-23.
^ "Fact Sheet: Fentanyl and Synthetic Opioids" (PDF). Drug Policy Alliance. September 2016. Retrieved 30 October 2018.
^ "Laws Learn about the laws concerning opioids from the 1800s until today". The National Alliance of Advocates for Buprenorphine Treatment. Retrieved 30 October 2018.
^ White, William L. "The Early Criminalization of Narcotic Addiction" (PDF). William White Papers. Retrieved 30 October 2018.
^ Mars, Sarah (2003). "Heroin Addiction Care and Control: the British System 1916 to 1984". J R Soc Med. 96 (2): 99–100. PMC 539406.
^ ab Jacobs, Harrison (May 26, 2016). "This one-paragraph letter may have launched the opioid epidemic". Business Insider. Retrieved 30 October 2018.
^ Portenoy, RK; Foley, KM (May 1986). "Chronic use of opioid analgesics in non-malignant pain: report of 38 cases". Pain. 25 (2): 171–86. PMID 2873550.
^ abc Meldrum, Marcia L. (August 2016). "The Ongoing Opioid Prescription Epidemic: Historical Context". American Journal of Public Health. 106 (8): 1365–1366. doi:10.2105/AJPH.2016.303297. PMC 4940677. PMID 27400351.
^ Quinones, Sam (2015). Dreamland : the true tale of America's opiate epidemic. Bloomsbury Press. ISBN 9781620402511.
^ Dowell, Deborah; Haegerich, Tamara M.; Chou, Roger (18 March 2016). "CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016". MMWR. Recommendations and Reports. 65 (1): 1–49. doi:10.15585/mmwr.rr6501e1. PMID 26987082.
^ "opioid: definition of opioid in Oxford dictionary (American English) (US)". www.oxforddictionaries.com. Retrieved 2016-02-14.Opioid: 1950s: from opium + -oid.
^ Wikler, A.; Martin, W. R.; Pescor, F. T.; Eades, C. G. (1963-10-24). "Factors regulating oral consumption of an opioid (etonitazene) by morphine-addicted rats". Psychopharmacologia. 5: 55–76. doi:10.1007/bf00405575. PMID 14082382.In this paper, the term, 'opioid', is used in the sense originally proposed by DR. GEORGE H. ACHESON (personal communication) to refer to any chemical compound with morphine-like activities.
^ Martin WR (1967). "Opioid antagonists". Pharmacol. Rev. 19 (4): 463–521. PMID 4867058.
^ Mehdi B (2008). "Opioid analgesics and antagonists". In Seth SD, Seth V. Textbook Of Pharmacology. Elsevier India. p. III.137. ISBN 9788131211588.CS1 maint: Uses editors parameter (link)
^ "Epidemic: Responding to America's Prescription Drug Abuse Crisis" (PDF). The White House. 2011. Archived from the original (PDF) on 2012-03-03.
^ "First Do No Harm: Responding to Canada's Prescription Drug Crisis" (PDF). March 2013. Retrieved March 8, 2017.
^ "UK: Task Force offers ideas for opioid addiction solutions". Delhidailynews.com. 2014-06-11. Retrieved 2016-01-07.
^ Allison Kite (29 April 2018). "Every state but Missouri has opioid drug tracking. Why are senators against it?". The Kansas City Star. Retrieved 5 November 2018.
^ Rutkow Lainie; et al. (2015). "Most primary care physicians are aware of prescription drug monitoring programs, but many find the data difficult to access". Health Affairs. 34 (3): 484–492. doi:10.1377/hlthaff.2014.1085. PMID 25732500.CS1 maint: Explicit use of et al. (link)
^ Matthew Perrone, Associated Press (2015-12-20). "Painkiller politics: Effort to curb prescribing under fire". Philly.com. Retrieved 2016-01-07.
^ Dowell, Deborah; Haegerich, Tamara; Chou, Roger (March 15, 2016). "CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016". JAMA. 315 (15): 1624–45. doi:10.1001/jama.2016.1464. PMID 26977696. Retrieved March 18, 2016.
^ Canan, Chelsea; Polinski, Jennifer M; Alexander, G Caleb; Kowal, Mary K; Brennan, Troyen A; Shrank, William H (2017-07-18). "Automatable algorithms to identify nonmedical opioid use using electronic data: a systematic review". Journal of the American Medical Informatics Association. 24 (6): 1204–1210. doi:10.1093/jamia/ocx066. ISSN 1067-5027. PMID 29016967.
^ Joel Achenbach, John Wagner, Lenny Bernstein. "Trump says opioid crisis is a national emergency, pledges more money and attention". Washington Post. Retrieved 2017-08-11.CS1 maint: Multiple names: authors list (link)
^ "Feasibility Study on Opium Licensing in Afghanistan for the Production of Morphine and Other Essential Medicines". ICoS. September 2005.
^ "Assuring Availability of Opioid Analgesics" (PDF). World Health Organization. Archived from the original (PDF) on 1 December 2009.
^ Ghelardini, Carla; Di Cesare Mannelli, Lorenzo; Bianchi, Enrica (2015). "The pharmacological basis of opioids". Clinical Cases in Mineral and Bone Metabolism. 12 (3): 219–221. doi:10.11138/ccmbm/2015.12.3.219. ISSN 1724-8914. PMC 4708964. PMID 26811699.The opioid effects transcending analgesia include sedation, respiratory depression, constipation and a strong sense of euphoria.
^ Barrett SP, Meisner JR, Stewart SH (November 2008). "What constitutes prescription drug misuse? Problems and pitfalls of current conceptualizations" (PDF). Curr Drug Abuse Rev. 1 (3): 255–62. doi:10.2174/1874473710801030255. PMID 19630724. Archived from the original (PDF) on 15 June 2010.
^ McCabe SE, Boyd CJ, Teter CJ (June 2009). "Subtypes of nonmedical prescription drug misuse". Drug Alcohol Depend. 102 (1–3): 63–70. doi:10.1016/j.drugalcdep.2009.01.007. PMC 2975029. PMID 19278795.
^ "Prescription Opioid Overdose Data | Drug Overdose | CDC Injury Center". www.cdc.gov. 2018-08-31. Retrieved 17 January 2017.
^ "Esters of Morphine". United Nations Office on Drugs and Crime. 1953. Retrieved 10 March 2012.
^ "Esters of Morphine Opioids". eOpiates. 2014-05-28. Retrieved 2016-02-12.
^ Raffa, Robert B.; Buschmann, Helmut; Christoph, Thomas; Eichenbaum, Gary; Englberger, Werner; Flores, Christopher M.; Hertrampf, Torsten; Kögel, Babette; Schiene, Klaus (2012-07-01). "Mechanistic and functional differentiation of tapentadol and tramadol". Expert Opinion on Pharmacotherapy. 13 (10): 1437–1449. doi:10.1517/14656566.2012.696097. ISSN 1744-7666. PMID 22698264.
^ Rojas-Corrales MO, Gibert-Rahola J, Micó JA (1998). "Tramadol induces antidepressant-type effects in mice". Life Sci. 63 (12): PL175–80. doi:10.1016/S0024-3205(98)00369-5. PMID 9749830.
^ Stein C, Schäfer M, Machelska H (2003). "Attacking pain at its source: new perspectives on opioids". Nature Medicine. 9 (8): 1003–1008. doi:10.1038/nm908. PMID 12894165.
^ Stein C, Lang LJ (2009). "Peripheral mechanisms of opioid analgesia". Curr Opin Pharmacol. 9 (1): 3–8. doi:10.1016/j.coph.2008.12.009. PMID 19157985.
^ Busch-Dienstfertig M, Stein C (2010). "Opioid receptors and opioid peptide-producing leukocytes in inflammatory pain-basic and therapeutic aspects". Brain Behav Immun. 24 (5): 683–694. doi:10.1016/j.bbi.2009.10.013. PMID 19879349.
^ Odell LR, Skopec J, McCluskey A (March 2008). "Isolation and identification of unique marker compounds from the Tasmanian poppy Papaver somniferum N. Implications for the identification of illicit heroin of Tasmanian origin". Forensic Sci. Int. 175 (2–3): 202–8. doi:10.1016/j.forsciint.2007.07.002. PMID 17765420.
External links
Opioid Withdrawal Symptoms—Information about Opioid and opiate withdrawal issues- World Health Organization guidelines for the availability and accessibility of controlled substances
- CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016
- Reference list to the previous publication
- Links to all language versions of the previous publication
- Video: Opioid side effects (Vimeo) (YouTube)—A short educational film about the practical management of opioid side effects.