Ribosomal RNA
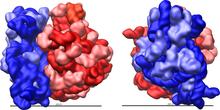
Three-dimensional views of the ribosome, showing rRNA in dark blue (small subunit) and dark red (large subunit). Lighter colors represent ribosomal proteins.
Ribosomal ribonucleic acid (rRNA) is the RNA component of the ribosome, and is essential for protein synthesis in all living organisms. It constitutes the predominant material within the ribosome, which is approximately 60% rRNA and 40% protein by weight, or 3/5 of ribosome mass. Ribosomes contain two major rRNAs and 50 or more proteins. The ribosomal RNAs form two subunits, the large subunit (LSU) and small subunit (SSU). The LSU rRNA acts as a ribozyme, catalyzing peptide bond formation. The SSU and LSU rRNA sequences are widely used for working out evolutionary relationships among organisms, since they are of ancient origin and are found in all known forms of life.
Contents
1 Structure
2 Subunits and ribosomal RNA genes
2.1 In prokaryotes
2.2 In eukaryotes
3 Transcription
3.1 In prokaryotes
3.2 In eukaryotes
4 Translation
5 Importance
6 Genes coding for ribosomal proteins
7 See also
8 References
9 External links
Structure
The ribosomal RNAs complex with proteins to form two subunits, the large subunit (LSU) and small subunit (SSU). During translation, mRNA is sandwiched between the small and large subunits, and the ribosome catalyzes the formation of a peptide bond between the two amino acids that are contained in the rRNA.
A ribosome also has three binding sites called A, P, and E.
- The A site in the ribosome binds to an aminoacyl-tRNA (a tRNA bound to an amino acid).
- The amino (NH2) group of the aminoacyl-tRNA, which contains the new amino acid, attacks the ester linkage of peptidyl-tRNA (contained within the P site), which contains the last amino acid of the growing chain, forming a new peptide bond. This reaction is catalyzed by peptidyl transferase.
- The tRNA that was holding onto the last amino acid is moved to the E site, and what used to be the aminoacyl-tRNA is the peptidyl-tRNA.
A single mRNA can be translated simultaneously by multiple ribosomes.
Subunits and ribosomal RNA genes
Both prokaryotic and eukaryotic ribosomes can be broken down into two subunits (the S in 16S represents Svedberg units), nt= length in nucleotides of the respective rRNAs, for exemplary species Escherichia coli (prokaryote) and human (eukaryote):
| Type | Size | Large subunit (LSU rRNA) | Small subunit (SSU rRNA) |
| prokaryotic | 70S | 50S (5S : 120 nt, 23S : 2906 nt) | 30S (16S : 1542 nt) |
| eukaryotic | 80S | 60S (5S : 121 nt,[1]5.8S : 156 nt,[2]28S : 5070 nt[3]) | 40S (18S : 1869 nt[4]) |
Note that the S units of the subunits (or the rRNAs) cannot simply be added because they represent measures of sedimentation rate rather than of mass. The sedimentation rate of each subunit is affected by its shape, as well as by its mass. The nt units can be added as these represent the integer number of units in the linear rRNA polymers (for example, the total length of the human rRNA = 7216 nt).
In prokaryotes
In prokaryotes a small 30S ribosomal subunit contains the 16S ribosomal RNA.
The large 50S ribosomal subunit contains two rRNA species (the 5S and 23S ribosomal RNAs).
Bacterial 16S ribosomal RNA, 23S ribosomal RNA, and 5S rRNA genes are typically organized as a co-transcribed operon.
There may be one or more copies of the operon dispersed in the genome (for example, Escherichia coli has seven).
Archaea contains either a single rDNA operon or multiple copies of the operon.
The 3' end of the 16S ribosomal RNA (in a ribosome) binds to a sequence on the 5' end of mRNA called the Shine-Dalgarno sequence.
In eukaryotes

Small subunit ribosomal RNA, 5' domain taken from the Rfam database. This example is RF00177
In contrast, eukaryotes generally have many copies of the rRNA genes organized in tandem repeats. In humans, approximately 300–400 repeats are present in five clusters, located on chromosomes 13 (RNR1), 14 (RNR2), 15 (RNR3), 21 (RNR4) and 22 (RNR5). Sequence variation in rRNA has been observed both within and between human individuals and certain variants are expressed in a tissue-specific manner in mice.[5] Because of their special structure and transcription behaviour, rRNA gene clusters are commonly called "ribosomal DNA" (note that the term seems to imply that ribosomes contain DNA, which is not the case).
The 18S rRNA in most eukaryotes is in the small ribosomal subunit, and the large subunit contains three rRNA species (the 5S, 5.8S and 28S in mammals, 25S in plants, rRNAs).
Mammalian cells have 2 mitochondrial (12S and 16S) rRNA molecules and 4 types of cytoplasmic rRNA (the 28S, 5.8S, 18S, and 5S subunits). The 28S, 5.8S, and 18S rRNAs are encoded by a single transcription unit (45S) separated by 2 internally transcribed spacers. The 45S rDNA is organized into 5 clusters (each has 30–40 repeats) on chromosomes 13, 14, 15, 21, and 22. These are transcribed by RNA polymerase I. 5S occurs in tandem arrays (~200–300 true 5S genes and many dispersed pseudogenes), the largest one on the chromosome 1q41-42. 5S rRNA is transcribed by RNA polymerase III.
The tertiary structure of the small subunit ribosomal RNA (SSU rRNA) has been resolved by X-ray crystallography.[6] The secondary structure of SSU rRNA contains 4 distinct domains—the 5', central, 3' major and 3' minor domains. A model of the secondary structure for the 5' domain (500-800 nucleotides) is shown.
Transcription
In prokaryotes
In prokaryotic cells, each rRNA gene or operon is transcribed into a single RNA precursor that includes 16S, 23S, 5S rRNA and tRNA sequences along with transcribed spacers. The RNA processing then begins before the transcription is complete. During processing reactions, the rRNAs and tRNAs are released as separate molecules.[7]
In eukaryotes
The genes coding for 18S, 28S and 5.8S rRNA, located in the nucleolus organizer region, are transcribed into a large pre-rRNA molecule by RNA polymerase I. Each large pre-rRNA contains 18S, 28S and 5.8S sequences which are separated by external and internal transcribed spacer sequences. During processing reactions, the 18S, 28S and 5.8S rRNA are released as individual molecules. Processing reactions involve exo- and endo-nucleolytic cleavages guided by snoRNA (small nucleolar RNAs) in complex with proteins. The genes for 5S rRNA are located inside the nucleolus and are transcribed into pre-5S rRNA by RNA polymerase III [8]. The pre-5S rRNA enters the nucleolus for processing and assembly with 28S and 5.8S rRNA to form the large subunit. 18S rRNA forms the small ribosomal subunits by combining with ribosomal proteins.[9][10]
Translation
Translation is the net effect of proteins being synthesized by ribosomes, from a copy (mRNA) of the DNA template in the nucleus. One of the components of the ribosome (16S rRNA) base pairs complementary to a Shine–Dalgarno sequence upstream of the start codon in mRNA.
Importance
Ribosomal RNA characteristics are important in evolution, thus taxonomy, and medicine.
- rRNA is one of only a few gene products present in all cells.[11] For this reason, genes that encode the rRNA (rDNA) are sequenced to identify an organism's taxonomic group, calculate related groups, and estimate rates of species divergence.[12] As a result, many thousands of rRNA sequences are known and stored in specialized databases such as RDP-II[13] and SILVA.[14]
- rRNA is the target of numerous clinically relevant antibiotics: chloramphenicol, erythromycin, kasugamycin, micrococcin, paromomycin, ricin, alpha-sarcin, spectinomycin, streptomycin, and thiostrepton.
- rRNA have been shown to be the origin of species-specific microRNAs, like miR-663 in humans and miR-712 in mouse. These miRNAs originate from the internal transcribed spacers of the rRNA.[15]
Genes coding for ribosomal proteins
These denote genes encoding for the proteins of the ribosome and are transcribed as mRNA, not rRNA.
RPL1, RPL2, RPL3, RPL4, RPL5, RPL6, RPL7, RPL8, RPL9, RPL10, RPL11, RPL12, RPL13, RPL14, RPL15, RPL16, RPL17, RPL18, RPL19, RPL20, RPL21, RPL22, RPL23, RPL24, RPL25, RPL26, RPL27, RPL28, RPL29, RPL30, RPL31, RPL32, RPL33, RPL34, RPL35, RPL36, RPL37, RPL38, RPL39, RPL40, RPL41
MRPL1, MRPL2, MRPL3, MRPL4, MRPL5, MRPL6, MRPL7, MRPL8, MRPL9, MRPL10, MRPL11, MRPL12, MRPL13, MRPL14, MRPL15, MRPL16, MRPL17, MRPL18, MRPL19, MRPL20, MRPL21, MRPL22, MRPL23, MRPL24, MRPL25, MRPL26, MRPL27, MRPL28, MRPL29, MRPL30, MRPL31, MRPL32, MRPL33, MRPL34, MRPL35, MRPL36, MRPL37, MRPL38, MRPL39, MRPL40, MRPL41, MRPL42
RPS1, RPS2, RPS3, RPS4, RPS5, RPS6, RPS7, RPS8, RPS9, RPS10, RPS11, RPS12, RPS13, RPS14, RPS15, RPS16, RPS17, RPS18, RPS19, RPS20, RPS21, RPS22, RPS23, RPS24, RPS25, RPS26, RPS27, RPS28, RPS29
MRPS1, MRPS2, MRPS3, MRPS4, MRPS5, MRPS6, MRPS7, MRPS8, MRPS9, MRPS10, MRPS11, MRPS12, MRPS13, MRPS14, MRPS15, MRPS16, MRPS17, MRPS18, MRPS19, MRPS20, MRPS21, MRPS22, MRPS23, MRPS24, MRPS25, MRPS26, MRPS27, MRPS28, MRPS29, MRPS30, MRPS31, MRPS32, MRPS33, MRPS34, MRPS35
See also
- Ribotyping
Diazaborine, a maturation inhibitor of rRNAs for the large ribosomal subunit
References
^ "Homo sapiens 5S ribosomal RNA"..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ "Homo sapiens 5.8S ribosomal RNA".
^ "Homo sapiens 28S ribosomal RNA".
^ "Homo sapiens 18S ribosomal RNA".
^ Parks, MM; Kurylo CM; Dass RA; Bojmar L; Lyden D; Vincent CT; Blanchard SC (2018). "Variant ribosomal RNA alleles are conserved and exhibit tissue-specific expression". Science Advances. 4 (2). doi:10.1126/sciadv.aao0665.
^ Yusupov MM, Yusupova GZ, Baucom A, et al. (2001). "Crystal structure of the ribosome at 5.5 A resolution". Science. 292 (5518): 883–96. doi:10.1126/science.1060089. PMID 11283358.
^ Wolfe, Stephen. Molecular and Cellular Biology. ISBN 978-0534124083.
^ Engelke, David R.; Good, Paul D.; Haeusler, Rebecca A.; Thompson, Martin (2003-11-21). "Nucleolar Clustering of Dispersed tRNA Genes". Science. 302 (5649): 1399–1401. doi:10.1126/science.1089814. ISSN 1095-9203. PMID 14631041.
^ "rRNA synthesis and processing".
^ Wolfe, Stephen. Molecular and Cellular Biology. ISBN 978-0534124083.
^ Smit S, Widmann J, Knight R (2007). "Evolutionary rates vary among rRNA structural elements". Nucleic Acids Res. 35 (10): 3339–54. doi:10.1093/nar/gkm101. PMC 1904297. PMID 17468501.
^ Meyer, Achim; Todt, Christiane; Mikkelsen, Nina T; Lieb, Bernhard (2010). "Fast evolving 18S rRNA sequences from Solenogastres (Mollusca) resist standard PCR amplification and give new insights into mollusk substitution rate heterogeneity". BMC Evolutionary Biology. 10 (1): 70. doi:10.1186/1471-2148-10-70. ISSN 1471-2148. Retrieved 27 July 2018.
^ Cole, JR; Chai B; Marsh TL; Farris RJ; Wang Q; Kulam SA; Chandra S; McGarrell DM; Schmidt TM; Garrity GM; Tiedje JM (2003). "The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy". Nucleic Acids Res. 31 (1): 442–3. doi:10.1093/nar/gkg039. PMC 165486. PMID 12520046.
^ Pruesse, E; Quast C; Knittel K; Fuchs BM; Ludwig W; Peplies J; Gloeckner FO (2007). "SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB". Nucleic Acids Res. 35 (1): 7188–7196. doi:10.1093/nar/gkm864. PMC 2175337. PMID 17947321.
^ The atypical mechanosensitive microRNA-712 derived from pre-ribosomal RNA induces endothelial inflammation and atherosclerosis Nature Communications, 2013 doi:10.1038/ncomms4000
External links
- 16S rRNA, BioMineWiki
- 16S rRNA, one of the most important rRNAs
- Ribosomal Database Project II
Ribosomal+RNA at the US National Library of Medicine Medical Subject Headings (MeSH)
SILVA rRNA Database Project (also includes Eukaryotes (18S) and LSU (23S/28S))- Video: rRNA: sequence, function & synthesis
