Capsid

Schematic of a cytomegalovirus

Illustration of geometric model changing between two possible capsids. A similar change of size has been observed as the result of a single amino-acid mutation[1]
A capsid is the protein shell of a virus. It consists of several oligomeric structural subunits made of protein called protomers. The observable 3-dimensional morphological subunits, which may or may not correspond to individual proteins, are called capsomeres. The capsid encloses the genetic material of the virus.
Capsids are broadly classified according to their structure. The majority of viruses have capsids with either helical or icosahedral[2][3] structure. Some viruses, such as bacteriophages, have developed more complicated structures due to constraints of elasticity and electrostatics.[4] The icosahedral shape, which has 20 equilateral triangular faces, approximates a sphere, while the helical shape resembles the shape of a spring, taking the space of a cylinder but not being a cylinder itself.[5] The capsid faces may consist of one or more proteins. For example, the foot-and-mouth disease virus capsid has faces consisting of three proteins named VP1–3.[6]
Some viruses are enveloped, meaning that the capsid is coated with a lipid membrane known as the viral envelope. The envelope is acquired by the capsid from an intracellular membrane in the virus' host; examples include the inner nuclear membrane, the golgi membrane, and the cell's outer membrane.[7]
Once the virus has infected a cell and begins replicating itself, new capsid subunits are synthesized using the protein biosynthesis mechanism of the cell. In some viruses, including those with helical capsids and especially those with RNA genomes, the capsid proteins co-assemble with their genomes. In other viruses, especially more complex viruses with double-stranded DNA genomes, the capsid proteins assemble into empty precursor procapsids that includes a specialized portal structure at one vertex. Through this portal, viral DNA is translocated into the capsid.[8]
Structural analyses of major capsid protein (MCP) architectures have been used to categorise viruses into lineages. For example, the bacteriophage PRD1, the algal virus Paramecium bursaria Chlorella virus (PBCV-1), mimivirus and the mammalian adenovirus have been placed in the same lineage, whereas tailed, double-stranded DNA bacteriophages (Caudovirales) and herpesvirus belong to a second lineage.[9][10]
Contents
1 Specific shapes
1.1 Icosahedral
1.2 Prolate
1.3 Helical
2 Functions
3 Chemical properties
4 Origin and evolution
5 See also
6 References
Specific shapes
Icosahedral

Icosahedral capsid of an adenovirus

Virus capsid T-numbers
The icosahedral structure is extremely common among viruses. The icosahedron consists of 20 triangular faces delimited by 12 fivefold vertexes and consists of 60 asymmetric units. Thus, an icosahedral virus is made of 60N protein subunits. The number and arrangement of capsomeres in an icosahedral capsid can be classified using the "quasi-equivalence principle" proposed by Donald Caspar and Aaron Klug.[11] Like the Goldberg polyhedra, an icosahedral structure can be regarded as being constructed from pentamers and hexamers. The structures can be indexed by two integers h and k, with h≥1displaystyle hgeq 1

- T=h2+h⋅k+k2displaystyle T=h^2+hcdot k+k^2
In this scheme, icosahedral capsids contain 12 pentamers plus 10(T − 1) hexamers. [12][13] The T-number is representative of the size and complexity of the capsids.[14] Geometric examples for many values of h, k, and T can be found at List of geodesic polyhedra and Goldberg polyhedra.
Many exceptions to this rule exist: For example, the polyomaviruses and papillomaviruses have pentamers instead of hexamers in hexavalent positions on a quasi-T=7 lattice. Members of the double-stranded RNA virus lineage, including reovirus, rotavirus and bacteriophage φ6 have capsids built of 120 copies of capsid protein, corresponding to a "T=2" capsid, or arguably a T=1 capsid with a dimer in the asymmetric unit. Similarly, many small viruses have a pseudo-T=3 (or P=3) capsid, which is organized according to a T=3 lattice, but with distinct polypeptides occupying the three quasi-equivalent positions [15]
T-numbers can be represented in different ways, for example T = 1 can only be represented as an icosahedron or a dodecahedron and, depending on the type of quasi-symmetry, T = 3 can be presented as a truncated dodecahedron, an icosidodecahedron, or a truncated icosahedron and their respective duals a triakis icosahedron, a rhombic triacontahedron, or a pentakis dodecahedron.[16][clarification needed]
Prolate
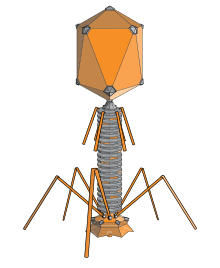
The prolate structure of a typical head on a bacteriophage
An elongated icosahedron is a common shape for the heads of bacteriophages. Such a structure is composed of a cylinder with a cap at either end. The cylinder is composed of 10 elongated triangular faces. The Q number (or Tmid), which can be any positive integer[17], specifies the number of triangles, composed of asymmetric subunits, that make up the 10 triangles of the cylinder. The caps are classified by the T (or Tend) number.[18]
Helical

3D model of a helical capsid structure of a virus
Many rod-shaped and filamentous plant viruses have capsids with helical symmetry.[19] The helical structure can be described as a set of n 1-D molecular helices related by an n-fold axial symmetry.[20] The helical transformation are classified into two categories: one-dimensional and two-dimensional helical systems.[20] Creating an entire helical structure relies on a set of translational and rotational matrices which are coded in the protein data bank.[20] Helical symmetry is given by the formula P = μ x ρ, where μ is the number of structural units per turn of the helix, ρ is the axial rise per unit and P is the pitch of the helix. The structure is said to be open due to the characteristic that any volume can be enclosed by varying the length of the helix.[21] The most understood helical virus is the tobacco mosaic virus.[19] The virus is a single molecule of (+) strand RNA. Each coat protein on the interior of the helix bind three nucleotides of the RNA genome. Influenza A viruses differ by comprising multiple ribonucleoproteins, the viral NP protein organizes the RNA into a helical structure. The size is also different the tobacco mosaic virus has a 16.33 protein subunits per helical turn,[19] while the influenza A virus has a 28 amino acid tail loop.[citation needed]
Functions
The functions of the capsid are to:
- protect the genome,
- deliver the genome, and
- interact with the host.
The virus must assemble a stable, protective protein shell to protect the genome from lethal chemical and physical agents. These include forms of natural radiation, extremes of pH or temperature and proteolytic and nucleolytic enzymes. For non-enveloped viruses, the capsid itself may be involved in interaction with receptors on the host cell, leading to penetration of the host cell membrane and internalization of the capsid. Delivery of the genome occurs by subsequent uncoating or disassembly of the capsid and release of the genome into the cytoplasm, or by ejection of the genome through a specialized portal structure directly into the host cell nucleus.
Chemical properties
The viral particle must be metastable so that interactions can be readily reversed when entering and uncoating a new host cell. If it attains the minimum free energy state, in which its system is in a state of equilibrium, it will be unlikely - and energetically unfavorable - for the subunits of the capsid to reverse their molecular interactions that are associated with attachment and entry into the host cell.[22] Each subunit of the capsid has identical bonding contacts with its neighbors, and the two binding contacts are usually noncovalent. The non-covalent bonding holds the structural unit together. The reversible formation of non-covalent bonds between properly folded subunits leads to error-free assembly and minimizes free energy, while maintaining metastability.[21]
Origin and evolution
It has been suggested that many viral capsid proteins have evolved on multiple occasions from functionally diverse cellular proteins.[23] The recruitment of cellular proteins appears to have occurred at different stages of evolution, so that some cellular proteins were captured and refunctionalized prior to the divergence of cellular organisms into the three contemporary domains of life, whereas others were hijacked relatively recently. As a result, some capsid proteins are widespread in viruses infecting distantly related organisms (e.g., capsid proteins with the jelly-roll fold), whereas others are restricted to a particular group of viruses (e.g., capsid proteins of alphaviruses).[23]
See also
- Geodesic polyhedron
- Goldberg–Coxeter construction
- Fullerene#Other buckyballs
References
| Wikimedia Commons has media related to Capsid. |
^ Asensio MA, Morella NM, Jakobson CM, Hartman EC, Glasgow JE, Sankaran B, Zwart PH, Tullman-Ercek D (2016). "A Selection for Assembly Reveals That a Single Amino Acid Mutant of the Bacteriophage MS2 Coat Protein Forms a Smaller Virus-like Particle". Nano Lett. 16 (9): 5944–5950. doi:10.1021/acs.nanolett.6b02948. PMID 27549001..mw-parser-output cite.citationfont-style:inherit.mw-parser-output .citation qquotes:"""""""'""'".mw-parser-output .citation .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-limited a,.mw-parser-output .citation .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .citation .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-ws-icon abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/4/4c/Wikisource-logo.svg/12px-Wikisource-logo.svg.png")no-repeat;background-position:right .1em center.mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-maintdisplay:none;color:#33aa33;margin-left:0.3em.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ Lidmar J, Mirny L, Nelson DR (2003). "Virus shapes and buckling transitions in spherical shells". Phys. Rev. E. 68 (5): 051910. arXiv:cond-mat/0306741. Bibcode:2003PhRvE..68e1910L. doi:10.1103/PhysRevE.68.051910. PMID 14682823.
^ Vernizzi G, Olvera de la Cruz M (2007). "Faceting ionic shells into icosahedra via electrostatics". Proc. Natl. Acad. Sci. USA. 104 (47): 18382–18386. Bibcode:2007PNAS..10418382V. doi:10.1073/pnas.0703431104. PMC 2141786. PMID 18003933.
^ Vernizzi G, Sknepnek R, Olvera de la Cruz M (2011). "Platonic and Archimedean geometries in multicomponent elastic membranes". Proc. Natl. Acad. Sci. USA. 108 (11): 4292–4296. Bibcode:2011PNAS..108.4292V. doi:10.1073/pnas.1012872108. PMC 3060260. PMID 21368184.
^ Branden, Carl; Tooze, John (1991). Introduction to Protein Structure. New York: Garland. pp. 161–162. ISBN 978-0-8153-0270-4.
^ "Virus Structure (web-books.com)".
^ Alberts, Bruce; Bray, Dennis; Lewis, Julian; Raff, Martin; Roberts, Keith; Watson, James D. (1994). Molecular Biology of the Cell (4th ed.). p. 280.
^ Newcomb WW, Homa FL, Brown JC (August 2005). "Involvement of the Portal at an Early Step in Herpes Simplex Virus Capsid Assembly". Journal of Virology. 79 (16): 10540–6. doi:10.1128/JVI.79.16.10540-10546.2005. PMC 1182615. PMID 16051846.
^ Krupovic, M; Bamford, DH (2008). "Virus evolution: how far does the double beta-barrel viral lineage extend?". Nat Rev Microbiol. 6 (12): 941–8. doi:10.1038/nrmicro2033. PMID 19008892.
^ Khayat et al. classified Sulfolobus turreted icosahedral virus (STIV) and Laurinmäki et al. classified bacteriophage Bam35 – Proc. Natl. Acad. Sci. U.S.A. 103, 3669 (2006); 102, 18944 (2005); Structure 13, 1819 (2005)
^ Caspar, D. L. D.; Klug, A. (1962). "Physical Principles in the Construction of Regular Viruses". Cold Spring Harbor Symp. Quant. Biol. 27: 1–24. doi:10.1101/sqb.1962.027.001.005. PMID 14019094.
^ Carrillo-Tripp, M.; Shepherd, C. M.; Borelli, I. A.; Venkataraman, S.; Lander, G.; Natarajan, P.; Johnson, J. E.; Brooks, C. L.; Reddy, V. S. (2009). "T-number index". VIPERdb. 37 (Database issue): D436–D442. doi:10.1093/nar/gkn840. PMC 2686430. PMID 18981051. Retrieved March 17, 2011.
^ Johnson, J. E.; Speir, J.A. (2009). Desk Encyclopedia of General Virology. Boston: Academic Press. pp. 115–123. ISBN 978-0-12-375146-1.
^ Mannige RV, Brooks CL III (2010). "Periodic Table of Virus Capsids: Implications for Natural Selection and Design". PLoS ONE. 5 (3): e9423. Bibcode:2010PLoSO...5.9423M. doi:10.1371/journal.pone.0009423. PMC 2831995. PMID 20209096.
^ "Virusworld".
^ K. V. Damodaran; Vijay S. Reddy; John E. Johnson; Charles L. Brooks III (2002). "A General Method to Quantify Quasi-equivalence in Icosahedral Viruses". J. Mol. Biol. 324 (4): 723–737. doi:10.1016/S0022-2836(02)01138-5. PMID 12460573.
^ A. Luque; D. Reguera (2010). "The structure of elongated viral capsids". Biophys. J. 98 (12): P2993–3003. doi:10.1016/j.bpj.2010.02.051. PMC 2884239. PMID 20550912.
^ Casjens, S. (2009). Desk Encyclopedia of General Virology. Boston: Academic Press. pp. 167–174. ISBN 978-0-12-375146-1.
^ abc Yamada S, Matsuzawa T, Yamada K, Yoshioka S, Ono S, Hishinuma T (December 1986). "Modified inversion recovery method for nuclear magnetic resonance imaging". Sci Rep Res Inst Tohoku Univ Med. 33 (1–4): 9–15. PMID 3629216.
^ abc Aldrich RA (February 1987). "Children in cities—Seattle's KidsPlace program". Acta Paediatr Jpn. 29 (1): 84–90. doi:10.1111/j.1442-200x.1987.tb00013.x. PMID 3144854.
^ ab Racaniello, Vincent R.; Enquist, L. W. (2008). Principles of Virology, Vol. 1: Molecular Biology. Washington, D.C: ASM Press. ISBN 978-1-55581-479-3.
^ http://www.science.uwaterloo.ca/~cchieh/cact/applychem/gibbsenergy.html
^ ab Krupovic, M; Koonin, EV (2017). "Multiple origins of viral capsid proteins from cellular ancestors". Proc Natl Acad Sci U S A. 114 (12): E2401–E2410. doi:10.1073/pnas.1621061114. PMC 5373398. PMID 28265094.
Robert Williams The Geometrical Foundation of Natural Structure: A source book of Design, 1979, pp. 142–144, Figure 4-49,50,51 Custers of 12 spheres, 42 spheres, 92 spheres- Antony Pugh, Polyhedra: a visual approach, 1976, Chapter 6. The Geodesic Polyhedra of R. Buckminster Fuller and Related Polyhedra

