Prenatal development
Prenatal development (from Latin natalis, meaning 'relating to birth') is the process in which an embryo and later fetus develops during gestation. Prenatal development starts with fertilization, the first stage in embryogenesis which continues in fetal development until birth.
In human pregnancy, prenatal development, also known as antenatal development, is the development of the embryo following fertilization, and continued as fetal development. By the end of the tenth week of gestational age the embryo has acquired its basic form and is referred to as a fetus. The next period is that of fetal development where many organs become fully developed. This fetal period is described both topically (by organ) and chronologically (by time) with major occurrences being listed by gestational age.
In other animals the very early stages of embryogenesis are the same as those in humans. In later stages, development across all taxa of animals and the length of gestation vary.
Contents
1 Terminology
2 Fertilization
3 Development of the embryo
4 Development of the fetus
5 Development of organ systems
5.1 Fetal blood
5.1.1 Red blood cells
5.1.2 White blood cells
5.2 Fetal hormones
6 Cognitive development
7 Nutrition
8 Growth rate
9 Factors influencing development
9.1 Poverty
9.2 Mother's age
9.3 Drug use
9.4 Alcohol
9.5 Smoking and nicotine
9.6 Diseases
9.7 Mother's diet and physical health
9.8 Low birth weight
9.9 Stress
9.10 Environmental toxins
10 See also
11 References
12 Further reading
13 External links
Terminology

Stages during pregnancy. Embryogenesis is marked in green. Weeks and months are numbered by gestation.
Different terms are used to describe prenatal development, meaning development before birth. A term with the same meaning is the "antepartum" (from Latin ante "before" and parere "to give birth") Sometimes "antepartum" is however used to denote the period between the 24th/26th week of gestational age until birth, for example in antepartum hemorrhage.[1][2]
The perinatal period (from Greek peri, "about, around" and Latin nasci "to be born") is "around the time of birth". In developed countries and at facilities where expert neonatal care is available, it is considered from 22 completed weeks (usually about 154 days) of gestation (the time when birth weight is normally 500 g) to 7 completed days after birth.[3] In many of the developing countries the starting point of this period is considered 28 completed weeks of gestation (or weight more than 1000 g).[4]
Fertilization

A sperm fertilizing an ovum.
When semen is released into the vagina, the spermatozoa travel through the cervix and body of the uterus and into the Fallopian tubes. Fertilization of the egg cell (ovum), usually takes place in one of the Fallopian tubes. Many sperm are released with the possibility of just one sperm cell managing to adhere to and enter the thick protective shell-like layer surrounding the ovum. The first sperm that penetrates fully into the egg donates its genetic material (DNA). The egg then polarizes, repelling any additional sperm. The resulting combination is called a zygote, a new and genetically unique organism. The term "conception" refers variably to either fertilization or to formation of the conceptus after its implantation in the uterus, and this terminology is controversial.
Prior to fertilization, each ovum, as a gamete, contains half of the genetic material that will fuse with the male gamete, which carries the other half of the genetic material (DNA). The ovum only carries the X female sex chromosome whilst the sperm carries a single sex chromosome of either an X or a male Y chromosome. The resulting human zygote is similar to the majority of somatic cells because it contains two copies of the genome in a diploid set of chromosomes. One set of chromosomes came from the nucleus of the ovum and the second set from the nucleus of the sperm.
The zygote is male if the egg is fertilized by a sperm that carries a Y chromosome, and it is female if the egg is fertilized by a sperm that carries an X chromosome.[5] The Y chromosome contains a gene, SRY, which will switch on androgen production at a later stage, leading to the development of a male body type. In contrast, the mitochondrial genetic information of the zygote comes entirely from the mother via the ovum.
Development of the embryo
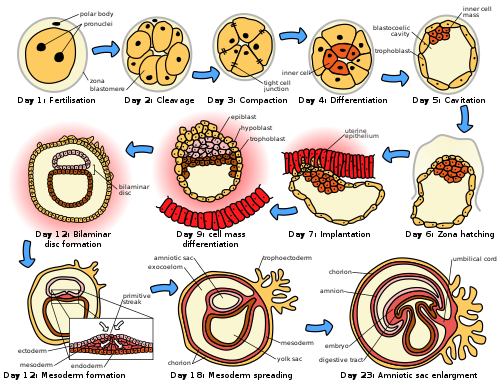
The initial stages of human embryogenesis.
The embryonic period in humans begins at fertilization (penetration of the egg by the sperm) and continues until the end of the 10th week of gestation (8th week by embryonic age). The period of two weeks from fertilization is also referred to as the germinal stage.
The embryo spends the next few days traveling down the Fallopian tube. It starts out as a single cell zygote and then divides several times to form a ball of cells called a morula. Further cellular division is accompanied by the formation of a small cavity between the cells. This stage is called a blastocyst. Up to this point there is no growth in the overall size of the embryo, as it is confined within a glycoprotein shell, known as the zona pellucida. Instead, each division produces successively smaller cells.
The blastocyst reaches the uterus at roughly the fifth day after fertilization. It is here that lysis of the zona pellucida occurs. This process is analogous to zona hatching, a term that refers to the emergence of the blastocyst from the zona pellucida, when incubated in vitro. This allows the trophectoderm cells of the blastocyst to come into contact with, and adhere to, the endometrial cells of the uterus. The trophectoderm will eventually give rise to extra-embryonic structures, such as the placenta and the membranes. The embryo becomes embedded in the endometrium in a process called implantation. In most successful pregnancies, the embryo implants 8 to 10 days after ovulation.[6] The embryo, the extra-embryonic membranes, and the placenta are collectively referred to as a conceptus, or the "products of conception".
Rapid growth occurs and the embryo's main features begin to take form. This process is called differentiation, which produces the varied cell types (such as blood cells, kidney cells, and nerve cells). A spontaneous abortion, or miscarriage, in the first trimester of pregnancy is usually[7] due to major genetic mistakes or abnormalities in the developing embryo. During this critical period (most of the first trimester), the developing embryo is also susceptible to toxic exposures, such as:
Alcohol, certain drugs, and other toxins that cause birth defects, such as fetal alcohol syndrome
Infection (such as rubella or cytomegalovirus)
Radiation from x-rays or radiation therapy
Nutritional deficiencies such as lack of folate which contributes to spina bifida
Development of the fetus
From the 10th week of gestation (8th week of development), the developing organism is called a fetus.
All major structures are already formed in the fetus, but they continue to grow and develop.
Since the precursors of all the major organs are created by this time, the fetal period is described both by organ and by a list of changes by weeks of gestational age.
Because the precursors of the organs are now formed, the fetus is not as sensitive to damage from environmental exposure as the embryo was. Instead, toxic exposure often causes physiological abnormalities or minor congenital malformation.
Development of organ systems
| This article is part of a series on the |
| Development of organ systems |
|---|
Nervous system |
Digestive system |
Reproductive system |
Urinary system |
Endocrine system |
Human development |
Circulatory system |
Development continues throughout the life of the embryo and fetus and through into life after birth. Significant changes occur to many systems in the period after birth as they adapt to life outside the uterus.
Fetal blood
Fetal hematopoiesis first takes place in the yolk sac. The function is transferred to liver by 10th week of gestation and to spleen and bone marrow beyond that. The total blood volume is about 125 ml/kg fetal body weight near term.
Red blood cells
Fetus produces megaloblastic red blood cells early in development, which become normoblastic near term. Life span of fetal RBCs is 80 days. Rh antigen appears at about 40 days of gestation.
White blood cells
Fetus starts producing leukocytes at 2 months gestation mainly from thymus and spleen. Lymphocytes derived from thymus are called T lymphocytes, whereas the ones derived from bone marrow are called B lymphocytes. Both these populations of lymphocytes have short-lived and long-lived groups. Short-lived T lymphocytes usually reside in thymus, bone marrow and spleen; whereas long-lived T lymphocytes reside in blood stream. Plasma cells are derived from B lymphocytes and their life in fetal blood is 0.5 to 2 days.
Fetal hormones
Thyroid gland is the first to develop in fetus at 4th week of gestation. Insulin secretion in fetus starts around 12th week of gestation.
Cognitive development
Initial knowledge of the effects of prenatal experience on later neuropsychological development originates from the Dutch Famine Study, which researched the cognitive development of individuals born after the Dutch famine of 1944–45.[8] The first studies focused on the consequences of the famine to cognitive development, including the prevalence of mental retardation.[9] Such studies predate David Barker's hypothesis about the association between the prenatal environment and the development of chronic conditions later in life.[10] The initial studies found no association between malnourishment and cognitive development[11], but later studies found associations between malnourishment and increased risk for schizophrenia[12], antisocial disorders[13], and affective disorders[14].
There is evidence that the acquisition of language beings in the prenatal stage. After 26 weeks of gestation, the peripheral auditory system is already fully formed.[15] Also, most low-frequency sounds (less than 300 HZ) can reach the fetal inner ear in the womb of mammals.[16] Those low-frequency sounds include pitch, rhythm, and phonetic information related to language.[17] Furthermore, there is evidence of language learning during gestation. Studies have indicated that fetuses react to and recognize differences between sounds.[18] Such ideas are further reinforced by the fact that newborns present a preference for their mother’s voice[19], present behavioral recognition of stories only heard during gestation[20], and (in monolingual mothers) present preference for their native language.[21] A more recent study with EEG demonstrated different brain activation in newborns hearing their native language compared to when they were presented with a different language, further supporting the idea that language learning starts while in gestation.[22]
Nutrition
The fetus passes through 3 phases of acquisition of nutrition from the mother:[23]
Absorption phase: Zygote is nourished by cellular cytoplasm and secretions in fallopian tubes and uterine cavity.
Histoplasmic transfer: After nidation and before establishment of uteroplacental circulation, fetal nutrition is derived from decidual cells and maternal blood pools that open up as a result of eroding activity of trophoblasts.
Hematotrophic phase: After third week of gestation, substances are transported passively via intervillous space.
Growth rate
Growth rate of fetus is linear up to 37 weeks of gestation, after which it plateaus.[23] The growth rate of an embryo and infant can be reflected as the weight per gestational age, and is often given as the weight put in relation to what would be expected by the gestational age. A baby born within the normal range of weight for that gestational age is known as appropriate for gestational age (AGA). An abnormally slow growth rate results in the infant being small for gestational age, and, on the other hand, an abnormally large growth rate results in the infant being large for gestational age. A slow growth rate and preterm birth are the two factors that can cause a low birth weight. Low birth weight (below 2000 grams) can ultimately increase the likelihood of schizophrenia by almost four times. [24]
The growth rate can be roughly correlated with the fundal height which can be estimated by abdominal palpation. More exact measurements can be performed with obstetric ultrasonography.
Factors influencing development
Intrauterine growth restriction is one of the causes of low birth weight associated with over half of neonatal deaths.[25]
Poverty
Poverty has been linked to poor prenatal care and has been an influence on prenatal development. Women in poverty are more likely to have children at a younger age, which results in low birth weight. Many of these expecting mothers have little education and are therefore less aware of the risks of smoking, drinking alcohol, and drug use – other factors that influence the growth rate of a fetus.
Mother's age
Women between the ages of 16 and 35 have a healthier environment for a fetus than women under 16 or over 35.[26] Women between this age gap are more likely to have fewer complications. Women over 35 are more inclined to have a longer labor period, which could potentially result in death of the mother or fetus. Women under 16 and over 35 have a higher risk of preterm labor (premature baby), and this risk increases for women in poverty, African Americans, and women who smoke. Young mothers are more likely to engage in high risk behaviors, such as using alcohol, drugs, or smoking, resulting in negative consequences for the fetus.[27] Premature babies from young mothers are more likely to have neurological defects that will influence their coping capabilities – irritability, trouble sleeping, constant crying for example. There is a risk of Down syndrome for infants born to those aged over 40 years. Young teenaged mothers (younger than 16) and mothers over 35 are more exposed to the risks of miscarriages, premature births, and birth defects.
Drug use
An estimated 5 percent of fetuses in the United States are exposed to illicit drug use during pregnancy.[28] Maternal drug use occurs when drugs ingested by the pregnant woman are metabolized in the placenta and then transmitted to the fetus. When using drugs (narcotics), there is a greater risk of birth defects, low birth weight, and a higher rate of death in infants or stillbirths. Drug use will influence extreme irritability, crying, and risk for SIDS once the fetus is born. The chemicals in drugs can cause an addiction in the babies once they are born. Marijuana will slow the fetal growth rate and can result in premature delivery. It can also lead to low birth weight, a shortened gestational period and complications in delivery. Heroin will cause interrupted fetal development, stillbirths, and can lead to numerous birth defects. Heroin can also result in premature delivery, creates a higher risk of miscarriages, result in facial abnormalities and head size, and create gastrointestinal abnormalities in the fetus. There is an increased risk for SIDS, dysfunction in the central nervous system, and neurological dysfunctions including tremors, sleep problems, and seizures. The fetus is also put at a great risk for low birth weight and respiratory problems. Cocaine use results in a smaller brain, which results in learning disabilities for the fetus. Cocaine puts the fetus at a higher risk of being stillborn or premature. Cocaine use also results in low birthweight, damage to the central nervous system, and motor dysfunction.
Alcohol
Maternal alcohol use leads to disruptions of the fetus's brain development, interferes with the fetus's cell development and organization, and affects the maturation of the central nervous system. Even small amounts of alcohol use can cause lower height, weight and head size at birth and higher aggressiveness and lower intelligence during childhood.[29]Fetal alcohol spectrum disorder is a developmental disorder that is a consequence of heavy alcohol intake by the mother during pregnancy. Children with FASD have a variety of distinctive facial features, heart problems, and cognitive problems such as developmental disabilities, attention difficulties, and memory deficits. [29]
Smoking and nicotine
When a mother smokes during pregnancy the fetus is exposed to nicotine, tar, and carbon monoxide. Nicotine results in less blood flow to the fetus because it constricts the blood vessels. Carbon monoxide reduces the oxygen flow to the fetus. The reduction of blood and oxygen flow may result in miscarriage, stillbirth, low birth weight, and premature births.[30] Exposure to secondhand smoke leads to higher risks of low birth weight and childhood cancer.[31]
Diseases
If a mother is infected with a disease, the placenta cannot always filter out pathogens. Viruses such as rubella, chicken pox, mumps, herpes, and human immunodeficiency (HIV) are associated with increased risk of miscarriage, low birth weight, and prematurity, physical malformations, and intellectual disabilities.[32] The human immunodeficiency virus can lead to acquired immune deficiency syndrome (AIDS). Untreated HIV infected expectant mothers pass the virus to their developing offspring between 10 and 20 percent of the time. [33]. Expectant mothers infected with bacterial or parasitic diseases may also pass the diseases on to the fetus. Examples of diseases include chlamydia, syphilis, tuberculosis, malaria, and toxoplasmosis. [34] One of the most common of these diseases is toxoplasmosis. A pregnant female may become infected through contact with contaminated soil, such as through gardening, eating raw or undercooked meat or unwashed vegetables or fruits, or contact with the feces of infected cats. If the exposure occurs during the first trimester, eye and brain damage may result. Exposure later in pregnancy may result in mild visual and cognitive impairments. [35]
Mother's diet and physical health
Adequate nutrition is needed for a healthy fetus. Mothers who gain less than 20 pounds during pregnancy are at increased risk for having a preterm or low birth weight infant.[36] . Iron and iodine are especially important during prenatal development. Mothers who are deficient in iron are at risk for having a preterm or low birth weight infant. [37] Iodine deficiencies increase the risk of miscarriage, stillbirth, and fetal brain abnormalities Adequate prenatal care gives an improved result in the newborn.[38]
Low birth weight
Low birth weight increases an infants risk of long-term growth and cognitive and language deficits. It also results in a shortened gestational period and can lead to prenatal complications.
Stress
Stress during pregnancy can impact the development of the embryo. Reilly (2017) states that stress can come from many forms of life events such as community, family, financial issues, and natural causes. While a woman is pregnant, stress from outside sources can take a toll on the growth in the womb that may affect the child’s learning and relationships when born. For instance, they may have behavioral problems and might be antisocial. The stress that the mother experiences affects the fetus and the fetus’ growth which can include the fetus’ nervous system (Reilly, 2017). Stress can also lead to low birth weight. Even after avoiding other factors like alcohol, drugs, and being healthy, stress can have its impacts whether families know it or not. Many women who deal with maternal stress do not seek treatment.
Similar to stress, Reilly stated that in recent studies, researchers have found that pregnant women who show depressive symptoms are not as attached and bonded to their child while it is in the womb (2017). [39]
Environmental toxins
Exposure to environmental toxins in pregnancy lead to higher rates of miscarriage, sterility, and birth defects. Toxins include fetal exposure to lead, mercury, and ethanol or hazardous environments. Prenatal exposure to mercury may lead to physical deformation, difficulty in chewing and swallowing, and poor motoric coordination.[40] Exposure to high levels of lead prenatally is related to prematurity, low birth weight, brain damage, and a variety of physical defects. [40] Exposure to persistent air pollution from traffic and smog may lead to reduced infant head size, low birth weight, increased infant death rates, impaired lung and immune system development. [41]
See also
- Fetal pig
References
^ patient.info » PatientPlus » Antepartum Haemorrhage Last Updated: 5 May 2009
^ The Royal Women’s Hospital > antepartum haemorrhage Archived 8 January 2010 at the Wayback Machine. Retrieved on Jan 13, 2009
^ Definitions and Indicators in Family Planning. Maternal & Child Health and Reproductive Health. By European Regional Office, World Health Organization. Revised March 1999 & January 2001. In turn citing: WHO Geneva, WHA20.19, WHA43.27, Article 23
^ Singh, Meharban (2010). Care of the Newborn. p. 7. Edition 7. .mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
ISBN 9788170820536
^ Schacter, Daniel (2009). "11-Development". Psychology Second Edition. United States of America: Worth Publishers. ISBN 978-1-4292-3719-2.
^ Wilcox AJ, Baird DD, Weinberg CR (1999). "Time of implantation of the conceptus and loss of pregnancy". N. Engl. J. Med. 340 (23): 1796–9. doi:10.1056/NEJM199906103402304. PMID 10362823.
^ Moore L. Keith. (2008). Before We Are Born: Essentials of Embryology and Birth Defects. Philadelphia, PA: Saunders/Elsevier. ISBN 978-1-4160-3705-7.
^ Henrichs, J. (2010). Prenatal determinants of early behavioral and cognitive development: The generation R study. Rotteram: Erasmus Universiteit.
^ Stein, Z., Susser, M., Saenger, G., & Marolla, F. (1972). Nutrition and mental performance. Science, 178(62),708-713.
^ Barker, D. J., Winter, P. D., Osmond, C., Margetts, B., & Simmonds, S. J. (1989). Weight in infancy and death from ischaemic heart disease. Lancet, 2(8663), 577-580.
^ Stein, Z., Susser, M., Saenger, G., & Marolla, F. (1972). Nutrition and mental performance. Science, 178(62),708-713.
^ Brown, A., Susser, E., Hoek, H., Neugebauer, R., Lin, S., & Gorman, J. (1996). Schizophrenia and affective disorders after prenatal famine. Biological Psychiatry, 39(7), 551. doi:10.1016/0006-3223(96)84122-9
^ Neugebauer, R., Hoek, H. W., & Susser, E. (1999). Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. Jama, 282(5), 455-462.
^ Brown, A. S., van Os, J., Driessens, C., Hoek, H. W., & Susser, E. S. (2000). Further evidence of relation between prenatal famine and major affective disorder. American Journal of Psychiatry, 157(2), 190-195.
^ Eisenberg, R. B. (1976). Auditory Competence in Early Life: The Roots of Communicate Behavior Baltimore: University Park Press.
^ Gerhardt, K. J., Otto, R., Abrams, R. M., Colle, J. J., Burchfield, D. J., and Peters, A. J. M. (1992). Cochlear microphones recorded from fetal and newborn sheep. Am. J. Otolaryngol. 13, 226–233.
^ Lecaneut, J. P., and Granier-Deferre, C. (1993). “Speech stimuli in the fetal environment,” in Developmental Neurocognition: Speech and Face Processing in the First Year of Life, eds B. De Boysson-Bardies, S. de Schonen, P. Jusczyk, P. MacNeilage, and J. Morton (Norwell, MA: Kluwer Academic Publishing), 237–248.
^ Kisilevsky, B. S., Hains, S. M. J., Lee, K., Xie, X., Ye, H. H., Zhang, K., and Wang, Z. (2003). Effects of experience on fetal voice recognition. Psychol. Sci. 14, 220–224.
^ DeCasper, A. J., and Fifer, W. P. (1980). Of human bonding: newborns prefer their mother’s voices. Science 208, 1174–1176.
^ DeCasper, A. J., and Spence, M. J. (1986). Prenatal maternal speech influences newborns’ perception of speech sounds. Infant Behav. Dev. 9, 133–150.
^ Moon, C., Cooper, R. P., and Fifer, W. P. (1993). Two-day-olds prefer their native language. Infant Behav. Dev. 16, 495–500.
^ May, L., Byers-Heinlein, K., Gervain, J., & Werker, J. F. (2011). Language and the Newborn Brain: Does Prenatal Language Experience Shape the Neonate Neural Response to Speech? Frontiers in Psychology, 2. doi:10.3389/fpsyg.2011.00222
^ ab Daftary, Shirish; Chakravarti, Sudip (2011). Manual of Obstetrics, 3rd Edition. Elsevier. pp. 1-16.
ISBN 9788131225561.
^ King, Suzanne; St-Hilaire, Annie; Heidkamp, David (2010). "Prenatal Factors in Schizophrenia". Current Directions in Psychological Science. 19 (4): 209–213. doi:10.1177/0963721410378360.
^ Lawn JE, Cousens S, Zupan J (2005). "4 million neonatal deaths: when? Where? Why?". The Lancet. 365 (9462): 891–900. doi:10.1016/s0140-6736(05)71048-5. PMID 15752534.
^ Centers for Disease Control (2018). "Pregnancy Complications". Centers for Disease Control. Archived from the original on 2018.
^ "About Teenage Pregnancy". Centers for Disease Control. 2018. Archived from the original on 2018.
^ Wendell, A. D. (2013). "Overview and epidemiology of substance abuse in pregnancy". Clinical Obstetrics & Gynecology. 56 (1): 91–96. doi:10.1097/GRF.0b013e31827feeb9. PMID 23314721.
^ ab Mattson, Sarah N.; Roesch, Scott C.; Fagerlund, Åse; Autti-Rämö, Ilona; Jones, Kenneth Lyons; May, Philip A.; Adnams, Colleen M.; Konovalova, Valentina; Riley, Edward P. (2010-06-21). "Toward a Neurobehavioral Profile of Fetal Alcohol Spectrum Disorders". Alcoholism: Clinical and Experimental Research. 34 (9): 1640–1650. doi:10.1111/j.1530-0277.2010.01250.x. ISSN 0145-6008. PMC 2946199. PMID 20569243.
^ Espy, Kimberly Andrews; Fang, Hua; Johnson, Craig; Stopp, Christian; Wiebe, Sandra A.; Respass, Jennifer (2011). "Prenatal tobacco exposure: Developmental outcomes in the neonatal period". Developmental Psychology. 47 (1): 153–169. doi:10.1037/a0020724. ISSN 1939-0599. PMC 3057676. PMID 21038943.
^ Rückinger, Simon; Beyerlein, Andreas; Jacobsen, Geir; von Kries, Rüdiger; Vik, Torstein (December 2010). "Growth in utero and body mass index at age 5years in children of smoking and non-smoking mothers". Early Human Development. 86 (12): 773–777. doi:10.1016/j.earlhumdev.2010.08.027. ISSN 0378-3782. PMID 20869819.
^ Waldorf, K. M. A. (2013). "Influence of infection during pregnancy on fetal development". Reproduction. 146 (5): 151–162. doi:10.1530/REP-13-0232. PMC 4060827. PMID 23884862.
^ [www.who.int/gho/publications/world_health_statistics/2014/en "World health statistics"] Check|archive-url=value (help). World Health Organization. 2014. Archived from [www.who.int/gho/publications/world_health_statistics/2014/en the original] Check|url=value (help) on 2014.
^ Diav-Citrin, O (2011). "Prenatal exposures associated with neurodevelopmental delay and disabilities". Developmental Disabilities. 17 (2): 71–84. doi:10.1002/ddrr.1102. PMID 23362027.
^ Jones, J. (2003). "Congenital toxoplasmosis". American Family Physician. 67 (10): 2131–2137. PMID 12776962.
^ Ehrenberg, H (2003). "Low maternal weight, failure to thrive in pregnancy, and adverse pregnancy outcomes". American Journal of Obstetrics and Gynecology. 189 (6): 1726–1730. PMID 14710105.
^ "Micronutrient deficiencies". World Health Organization. 2002.
^ "What is prenatal care and why is it important?". www.nichd.nih.gov.
^ Reilly, Nicole (2017). "Stress, depression and anxiety during pregnancy: How does it impact on children and how can we intervene early?". International Journal of Birth & Parent Education. 5 (1): 9–12.
^ ab Caserta, D (2013). "Heavy metals and placental fetal-maternal barrier: A mini review on the major concerns". European Review for Medical and Pharmacological Sciences. 17 (16): 2198–2206. PMID 23893187.
^ Proietti, E (2013). "Air pollution during pregnancy and neonatal outcome: A review". Journal of Aerosol Medicine and Pulmonary Drug Delivery. 26 (1): 9–23. doi:10.1089/jamp.2011.0932. PMID 22856675.
Further reading
MedlinePlus Encyclopedia Fetal development
Moore, Keith L. The Developing Human (3rd ed.). Philadelphia PA: W.B. Saunders Company.
Wilcox AJ, Baird DD, Weinberg CR (June 1999). "Time of implantation of the conceptus and loss of pregnancy". N. Engl. J. Med. 340 (23): 1796–9. doi:10.1056/NEJM199906103402304. PMID 10362823.
Ljunger E, Cnattingius S, Lundin C, Annerén G (November 2005). "Chromosomal anomalies in first-trimester miscarriages". Acta Obstet Gynecol Scand. 84 (11): 1103–7. doi:10.1111/j.0001-6349.2005.00882.x. PMID 16232180.
Newman, Barbara; Newman, Philip (10 March 2008). "The Period of Pregnancy and Prenatal Development". Development Through Life: A Psychosocial Approach. Cengage Learning. ISBN 978-0-495-55341-0.- "Prenatal Development - Prenatal Environmental Influences - Mother, Birth, Fetus, and Pregnancy." Social Issues Reference. Version Child Development Vol. 6. N.p., n.d. Web. 19 Nov. 2012. <http://social.jrank.org/pages/515/Prenatal-Development-Prenatal-Environmental-Influences.html>.
- Niedziocha, Laura. "The Effects Of Drugs And Alcohol On Fetal Development | LIVESTRONG.COM." LIVESTRONG.COM - Lose Weight & Get Fit with Diet, Nutrition & Fitness Tools | LIVESTRONG.COM. N.p., 4 Sept. 2011. Web. 19 Nov. 2012. <http://www.livestrong.com/article/535499-the-effects-of-drugs-and-alcohol-on-fetal-development/>.
Jaakkola, JJ; Gissler, M (January 2004). "Maternal smoking in pregnancy, fetal development, and childhood asthma". American Journal of Public Health. 94 (1): 136–40. doi:10.2105/ajph.94.1.136. PMC 1449839. PMID 14713711.
Gutbrod, T (1 May 2000). "Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison". Archives of Disease in Childhood: Fetal and Neonatal Edition. 82 (3): 208F–214. doi:10.1136/fn.82.3.F208. PMC 1721075. PMID 10794788.- Brady, Joanne P., Marc Posner, and Cynthia Lang. "Risk and Reality: The Implications of Prenatal Exposure to Alcohol and Other Drugs ." ASPE. N.p., n.d. Web. 19 Nov. 2012. <http://aspe.hhs.gov/hsp/cyp/drugkids.htm>.
Pregnancy Approach (eBook ed.). Lauren Lee. 2013.
External links
| Wikimedia Commons has media related to Embryology. |
Chart of human fetal development, U.S. National Library of Medicine (NLM)
U.K. Human Fertilisation and Embryology Authority (HFEA), regulatory agency overseeing the use of gametes and embryos in fertility treatment and research
