Sulfide
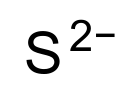 | |
| Names | |
|---|---|
Systematic IUPAC name Sulfanediide[1] (substitutive) Sulfide(2−)[1] (additive) | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
ChEBI |
|
ChemSpider |
|
PubChem CID |
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula | S2− |
Molar mass | 32.06 g·mol−1 |
Conjugate acid | Bisulfide |
| Related compounds | |
Other anions | Telluride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Infobox references | |
Sulfide (systematically named sulfanediide, and sulfide(2−)) (British English sulphide) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. It contributes no color to sulfide salts. As it is classified as a strong base, even dilute solutions of salts such as sodium sulfide (Na2S) are corrosive and can attack the skin. Sulfide is the simplest sulfur anion.
Contents
1 Nomenclature
2 Chemical properties
2.1 Chemical reactions
3 Metal derivatives
4 Geology
5 Corrosion induced by sulfide
6 Organic chemistry
7 Disulfides
8 Examples
9 Preparation
10 Safety
11 See also
12 References
Nomenclature
The systematic names sulfanediide and sulfide(2−), valid IUPAC names, are determined according to the substitutive and additive nomenclatures, respectively. However, the name sulfide is also used in compositional IUPAC nomenclature which does not take the nature of bonding involved. Examples of such naming are selenium disulfide and titanium sulfide, which contains no sulfide ions whatsoever.
Sulfide is also used non-systematically, to describe compounds which release hydrogen sulfide upon acidification, or a compound that otherwise incorporates sulfur in some form, such as dimethyl sulfide. "Hydrogen sulfide" is itself an example of a non-systematic name of this nature. However, it is also a trivial name, and the preferred IUPAC name for sulfane.
Chemical properties
It has been confirmed that the sulfide ion, S2−, does not exist even in hyper-concentrated aqueous alkaline solutions of Na2S.[2] Thus, the dissociation reaction
- SH− (aq) → S2− + H+
does not occur in aqueous solution at any concentration of sulphide. The sulphide ion, S2−, was previously reported to be undetectable at concentrations up to 5 M NaOH.[3] However, the sulfide ion may be produced when a solid is formed. For example cadmium sulphide precipitates in group 2 of qualitative analysis.
- H2S (g) + Cd2+ (aq) + 2OH− → CdS↓ (s) + 2H2O
Chemical reactions
Upon treatment with a standard acid, sulfide converts to hydrogen sulfide (H2S) and a metal salt. Oxidation of sulfide gives sulfur or sulfate. Metal sulfides react with nonmetals including iodine, bromine, and chlorine forming sulfur and metal salts.
- 8 MgS + 8 I2 → S8 + 8 MgI2
Sulfur can also be prepared from a sulfide and an appropriate oxidizer:
Metal derivatives
Aqueous solutions of transition metals cations react with sulfide sources (H2S, NaHS, Na2S) to precipitate solid sulfides. Such inorganic sulfides typically have very low solubility in water, and many are related to minerals with the same composition (see below). One famous example is the bright yellow species CdS or "cadmium yellow". The black tarnish formed on sterling silver is Ag2S. Such species are sometimes referred to as salts. In fact, the bonding in transition metal sulfides is highly covalent, which gives rise to their semiconductor properties, which in turn is related to the deep colors. Several have practical applications as pigments, in solar cells, and as catalysts. The fungus Aspergillus niger plays a role in the solubilization of heavy metal sulfides.[4]
Geology
Many important metal ores are sulfides.[5] Significant examples include: argentite (silver sulfide), cinnabar (mercury), galena (lead sulfide), molybdenite (molybdenum sulfide), pentlandite (nickel sulfide), realgar (arsenic sulfide), and stibnite (antimony), sphalerite (zinc sulfide), and pyrite (iron disulfide), and chalcopyrite (iron-copper sulfide).
Corrosion induced by sulfide
Dissolved free sulfides (H2S, HS− and S2−) are very aggressive species for the corrosion of many metals such as steel, stainless steel, and copper. Sulfides present in aqueous solution are responsible for stress corrosion cracking (SCC) of steel, and is also known as sulfide stress cracking. Corrosion is a major concern in many industrial installations processing sulfides: sulfide ore mills, deep oil wells, pipelines transporting soured oil, Kraft paper factories.
Microbially-induced corrosion (MIC) or biogenic sulfide corrosion are also caused by sulfate reducing bacteria producing sulfide that is emitted in the air and oxidized in sulfuric acid by sulfur oxidizing bacteria. Biogenic sulfuric acid reacts with sewerage materials and most generally causes mass loss, cracking of the sewer pipes and ultimately, structural collapse. This kind of deterioration is a major process affecting sewer systems worldwide and leading to very high rehabilitation costs.
Oxidation of sulfide can also form thiosulfate (S
2O2−
3) an intermediate species responsible for severe problems of pitting corrosion of steel and stainless steel while the medium is also acidified by the production of sulfuric acid when oxidation is more advanced.
Organic chemistry
In organic chemistry, "sulfide" usually refers to the linkage C–S–C, although the term thioether is less ambiguous. For example, the thioether dimethyl sulfide is CH3–S–CH3. Polyphenylene sulfide (see below) has the empirical formula C6H4S. Occasionally, the term sulfide refers to molecules containing the –SH functional group. For example, methyl sulfide can mean CH3–SH. The preferred descriptor for such SH-containing compounds is thiol or mercaptan, i.e. methanethiol, or methyl mercaptan.
Disulfides
Confusion arises from the different meanings of the term "disulfide". Molybdenum disulfide (MoS2) consists of separated sulfide centers, in association with molybdenum in the formal +4 oxidation state (that is, Mo4+ and two S2−). Iron disulfide (pyrite, FeS2) on the other hand consists of S2−
2, or −S–S− dianion, in association with divalent iron in the formal +2 oxidation state (ferrous ion: Fe2+). Dimethyldisulfide has the chemical binding CH3–S–S–CH3, whereas carbon disulfide has no S–S bond, being S=C=S (linear molecule analog to CO2). Most often in sulfur chemistry and in biochemistry, the disulfide term is commonly ascribed to the sulfur analogue of the peroxide –O–O– bond. The disulfide bond (–S–S–) plays a major role in the conformation of proteins and in the catalytic activity of enzymes.
Examples
| Formula | Melting point (°C) | Boiling point (°C) | CAS number | |
|---|---|---|---|---|
| H2S | Hydrogen sulfide is a very toxic and corrosive gas characterised by a typical odour of "rotten egg". | −85.7 | −60.20 | 7783-06-4 |
| CdS | Cadmium sulfide can be used in photocells. | 1750 | 1306-23-6 | |
Calcium polysulfide ("lime sulfur") is a traditional fungicide in gardening. | ||||
| CS2 | Carbon disulfide is sometimes used as a solvent in industrial chemistry. | −111.6 | 46 | 75-15-0 |
| PbS | Lead sulfide is used in infra-red sensors. | 1114 | 1314-87-0 | |
| MoS2 | Molybdenum disulfide, the mineral molybdenite, is used as a catalyst to remove sulfur from fossil fuels; also as lubricant for high-temperature and high-pressure applications. | 1317-33-5 | ||
| Cl–CH2CH2–S–CH2CH2–Cl | Sulfur mustard (mustard gas) is an organosulfide (thioether) that has been used as a chemical weapon in the First World War, the chloride on the molecule acts as a leaving group when in the presence of water and forms a thioether-alcohol and HCl. | 13–14 | 217 | 505-60-2 |
| Ag2S | Silver sulfide is formed on silver electrical contacts operating in an atmosphere rich in hydrogen sulfide. | 21548-73-2 | ||
| Na2S | Sodium sulfide is an important industrial chemical, used in manufacture of kraft paper, dyes, leather tanning, crude petroleum processing, treatment of heavy metal pollution, and others. | 920 | 1180 | 1313-82-2 |
| ZnS | Zinc sulfide is used for lenses and other optical devices in the infrared part of the spectrum. Zinc sulfide doped with silver is used in alpha detectors while zinc sulfide with traces of copper has applications in photoluminescent strips for emergency lighting and luminous watch dials. | 1185 | 1314-98-3 | |
| MeS | Several metal sulfides are used as pigments in art, although their use has declined somewhat due to their toxicity. Sulfide pigments include cadmium, mercury, and arsenic. | |||
| C6H4S | Polyphenylene sulfide is a polymer commonly called "Sulfar". Its repeating units are bonded together by sulfide (thioether) linkages. | 26125-40-6 25212-74-2 | ||
| SeS2 | Selenium disulfide is an antifungal used in anti-dandruff preparations, such as Selsun Blue. The presence of the highly toxic selenium in healthcare and cosmetics products represents a general health and environmental concern. | <100 | 7488-56-4 | |
| FeS2 | The crystal lattice of pyrite is made of iron disulfide, in which iron is divalent and present as ferrous ion (Fe2+). | 600 | 1317-66-4 |
Preparation
Sulfide compounds can be prepared in several different ways:[6]
- Direct combination of elements:
- Example: Fe(s) + S(s) → FeS(s)
- Reduction of a sulfate:
- Example: MgSO4(s) + 4C(s) → MgS(s) + 4CO(g)
- Precipitation of an insoluble sulfide:
- Example: M2+ + H2S(g) → MS(s) + 2H+(aq)
Safety
Many metal sulfides are so insoluble in water that they are probably not very toxic. Some metal sulfides, when exposed to a strong mineral acid, including gastric acids, will release toxic hydrogen sulfide.
Organic sulfides are highly flammable. When a sulfide burns it produces sulfur dioxide (SO2) gas.
Hydrogen sulfide, some of its salts, and almost all organic sulfides have a strong and putrid stench; rotting biomass releases these.
See also
- Dithionite
- Sulfate
- Sulfide stress cracking
- Sulfite
- Tetrathionate
- Thioether
- Thiosulfate
References
^ ab "sulfide(2−) (CHEBI:15138)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute..mw-parser-output cite.citationfont-style:inherit.mw-parser-output qquotes:"""""""'""'".mw-parser-output code.cs1-codecolor:inherit;background:inherit;border:inherit;padding:inherit.mw-parser-output .cs1-lock-free abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/6/65/Lock-green.svg/9px-Lock-green.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-limited a,.mw-parser-output .cs1-lock-registration abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/d/d6/Lock-gray-alt-2.svg/9px-Lock-gray-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-lock-subscription abackground:url("//upload.wikimedia.org/wikipedia/commons/thumb/a/aa/Lock-red-alt-2.svg/9px-Lock-red-alt-2.svg.png")no-repeat;background-position:right .1em center.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registrationcolor:#555.mw-parser-output .cs1-subscription span,.mw-parser-output .cs1-registration spanborder-bottom:1px dotted;cursor:help.mw-parser-output .cs1-hidden-errordisplay:none;font-size:100%.mw-parser-output .cs1-visible-errorfont-size:100%.mw-parser-output .cs1-subscription,.mw-parser-output .cs1-registration,.mw-parser-output .cs1-formatfont-size:95%.mw-parser-output .cs1-kern-left,.mw-parser-output .cs1-kern-wl-leftpadding-left:0.2em.mw-parser-output .cs1-kern-right,.mw-parser-output .cs1-kern-wl-rightpadding-right:0.2em
^ May, P.M.; Batka, D.; Hefter, G.; Könignberger, E.; Rowland, D. (2018). "Goodbye to S2-". Chem. Comm. doi:10.1039/c8cc00187a.
^ Meyer, B; Ward, K; Koshlap, K; Peter, L (1983). "Second dissociation constant of hydrogen sulfide". Inorganic Chemistry. 22: 2345. doi:10.1021/ic00158a027.
^ Harbhajan Singh. Mycoremediation: Fungal Bioremediation. p. 509.
^ Vaughan, D. J.; Craig, J. R. “Mineral chemistry of metal sulfides" Cambridge University Press, Cambridge: 1978.
ISBN 0-521-21489-0.
^ Atkins; Shriver (2010). Inorganic Chemistry (5th ed.). New York: W. H. Freeman & Co. p. 413.
